Published online Jul 14, 2005. doi: 10.3748/wjg.v11.i26.4032
Revised: October 2, 2004
Accepted: October 7, 2004
Published online: July 14, 2005
AIM: To assess the effects of ulcerogenic agents on actin cytoskeleton and cell motility and the contribution of oxidative stress.
METHODS: Rat gastric mucosal cell monolayers were cultured on coverslips. The cells were exposed, with or without allopurinol (2 mmol/L), for 15 min to ethanol (10-150 mL/L), ASA (1-20 mmol/L) or taurocholate (1-20 mmol/L), then the cells were processed for actin and vinculin staining. Cell migration after wounding was also measured.
RESULTS: Exposure to 10 mL/L ethanol caused divergence of zonula adherens-associated actin bundles of adjacent cells and decreased rate of migration. These actions were opposed by xanthine oxidase inhibitor allopurinol. Exposure to 50 mL/L ethanol induced degradation and divergence of zonula adherens-associated vinculin from adjacent cells, which was, again, partially reverted by allopurinol. With 1 mmol/L ASA actin filaments became shorter and thicker. However, higher concentrations (10, 20 mmol/L) of ASA returned microfilaments thinner and longer, and decreased rate of migration. Zonula adherens-associated actin bundles were moderately distorted with 10 mmol/L ASA and with 10 mmol/L taurocholate. Exposure to taurocholate provoked changes resembling those of ASA. Taurocholate 5-20 mmol/L decreased the rate of migration dose dependently. The effects of ASA and taurocholate were not prevented by allopurinol.
CONCLUSION: All ulcerogenic agents decreased the rate of migration dose dependently and induced divergence of zonula adherens-associated actin bundles of adjacent cells. In addition, ethanol and ASA caused degradation of actin cytoskeleton. Oxidative stress seems to underlie ethanol, but not ASA or taurocholate, induced cytoskeletal damage.
- Citation: Bidel S, Mustonen H, Khalighi-Sikaroudi G, Lehtonen E, Puolakkainen P, Kiviluoto T, Kivilaakso E. Effect of the ulcerogenic agents ethanol, acetylsalicylic acid and taurocholate on actin cytoskeleton and cell motility in cultured rat gastric mucosal cells. World J Gastroenterol 2005; 11(26): 4032-4039
- URL: https://www.wjgnet.com/1007-9327/full/v11/i26/4032.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i26.4032
The gastric mucosa is frequently exposed to different exogenous and endogenous ulcerative agents, such as ethanol, aspirin and taurocholate. These agents have been extensively investigated with methods, including biochemistry, morp-hology, electrophysiology, tissue permeability, etc., but the cellular mechanisms of injury are still poorly defined.
In digestive epithelia actin cytoskeleton is involved, for example, in organization of cytoplasm, maintenance of cell shape, generation and maintenance of epithelial polarity and in migration. The cytoplasm of epithelial cell is spatially and temporally organized by microfilaments, microtubules and intermediate filaments, which form a lattice-like intracellular meshwork[1,2].
The actin cytoskeleton is highly conserved in eukaryotic cells, including more than 70 categorized types of actin-binding proteins[3]. The human actin family includes α-, β-, and γ-actin, which share most of their amino acid sequences. The actin filaments are formed by polymerization of actin monomers. The polymeric actins are assembled into a filamentous network, which is regulated by actin-binding proteins. These proteins regulate, for example, polymerization, cross linking, nucleation and branching, anchoring, capping, membrane interaction, cell-extracellular matrix interaction, cell-cell interaction and contractility of actin filaments[4]. The actin filaments are bundled together by various actin cross-linking proteins to form complex three-dimensional structures. The filaments are extensively branched near the plasma membrane, focal adhesions, and adherens and tight junctions. Arp2/3 complex is involved in this branching[5]. Different components of the cytoskeleton are tightly involved in cell motility[6]. Actin based cell motility has several important tasks in epithelial cell functions, such as in secretory vesicle movement and cell migration during wound healing[7]. The cell migration starts with lamellipodia extension and formation of new focal adhesions, followed by the contraction of the cell body and detachment of the tail. Rho guanosine triphosphatases have a key role in regulating different phases of migration. Rac1 seems to be involved in initiation of migration[8] and in stimulation of lamellipodium extension[9], while Rho seems to contribute mainly to cell body contraction[6].
Epithelial cells have specialized mechanisms to enable cell-cell and cell-extracellular matrix adhesions. These connections are formed by transmembrane proteins, which bind to extracellular matrix or adjacent cells with their extracellular domains, while the intracellular domains bind to intracellular cytoskeleton via cytoplasmic adaptor proteins, many of which interact with the actin cytoskeleton[10]. Integrins mediate cellular adhesion to the extracellular matrix. They are linked to the actin cytoskeleton in focal adhesion complexes, which include a multitude of proteins, such as vinculin, talin, paxillin and calpain. These focal adhesions are involved in regulation of the cell migration and proliferation. The classical E-cadherins mediate Ca2+ dependent cell-cell adhesion through their extracellular Ca2+ binding repeats, while their cytoplasmic part binds to the actin cytoskeleton via β- and α-catenin. This complex is associated with a variety of other proteins, including vinculin, α-actinin, and paxillin. Zonula adherens is an E-cadherin based belt-like adherent junction just below the tight junctions encircling the epithelial cells adhered to each other. Along the cytoplasmic side of the zonula adherens runs a contractile bundle of actin filaments, which are associated to zonula adherens with a set of intracellular proteins, such as a-catenin, vinculin, -actinin and plakoglobin. Vinculin is a large protein with multiple binding sites to different adhesion related proteins, including actin, a-actinin, talin, paxillin, VASP, ponsin, vinexin and PKC[11,12]. Vinculin is thought to mediate the linkage between actin cytoskeleton and cadherins or integrins in cell-cell or cell-extracellular matrix adhesions. Recently vinculin has been shown to transiently associate with arp2/3 complex in the nascent focal complexes in the leading edge[13], possibly linking cellular protrusion and adhesion to extracellular matrix. Ethanol is known to cause oxidative stress in rat hepatocytes and in gastric mucosal cells[14,15]. Oxidative stress can cause various effects in the cell, such as oxidation and fragmentation of cellular proteins, peroxidations of membrane lipids, fragmentation of DNA and mitochondrial damage. Among the very early effects of oxidative stress might be disruption of the cytoskeleton[16].
The aim of the present study was to characterize the effects of different ulcerogenic agents on actin cytoskeleton, cell adhesion molecules and cell migration, and to investigate the possible role of oxidative stress in the cellular injury induced by them. This was accomplished by confocal and normal fluorescence imaging of actin and vinculin during exposure of the epithelial cells to the ulcerogenic agents. We also measured the rate of cell migration during exposure to the ulcerogenic agents to assess the functional state of the cytoskeleton. We further studied the possible role of reactive oxygen species in epithelial injury by inhibiting endogenous intracellular generation of superoxide radicals from purines with a xanthine oxidase inhibitor, allopurinol.
Immortal rat gastric mucosal cells (RGM-1, Riken Cell Bank, Riken, Japan)[17] were cultured on coverslips to confluent monolayers at 37°C in humidified atmosphere containing 50 mL/L CO2 in air. The medium used was an equal mixture of Ham’s F12 and Dulbeccos’s MEM supplemented with inactivated 200 mL/L fetal bovine serum, 100 U/mL penicillin, 100 µg/mL streptomycin and 0.125 µg/mL amphotericin.
The cells were exposed for 15 min to the following ulcerogenic agents: ethanol (10-150 mL/L), acetylsalicylic acid (ASA, 1-20 mmol/L) and taurocholate (1-20 mmol/L) at 37°C. In allopurinol experiments the samples were treated with 2 mmol/L allopurinol 24 h before and during the exposure of the ulcerogenic agent. Thereafter the cells were fixed with 35 mL/L paraformaldehyde in PBS for 15-20 min at room temperature and stained as described below.
Actin filaments and focal adhesion plaques were stained with phallotoxins conjugated to Alexa 488 fluorophore (Molecular Probes, A-12379). After fixation the cells were washed three times for 5-10 min with PBS at room temperature, permeabilized with 1 mL/L Triton-X, 1 mL/L bovine serum albumin in PBS and washed again three times for 5-10 min in PBS. Thereafter, the samples were incubated for 30-60 min in PBS with 80 mL/L bovine serum albumin and washed three times for 5-10 min with PBS and incubated overnight with the mouse monoclonal anti-vinculin (10 µg/mL, with 10 mL/L bovine serum albumin). The samples were washed three times for 5-10 min with PBS and were incubated with secondary antibody goat anti-mouse Alexa 568 (10 µg/mL) and Alexa 488 phalloidin (1:1 000) with 1 mL/L bovine serum albumin in PBS for 30 min at room temperature. Thereafter, the samples were washed three times for 5-10 min with PBS and mounted with DABCO containing mounting media. Confocal fluorescence microscopy was performed with a Leica TCS SP2 confocal microscope and normal fluorescence microscopy with an Olympus IX50 microscope.
RGM cells were cultured to confluency on plastic culture dishes as described above. In allopurinol series the confluent monolayers were treated with allopurinol after and 24 h before wounding. Artificial round-shaped wounds were created in the cell monolayer with a silicon tip, where after control (water) or ulcerogenic agents were added. The migration distance of the cells at the wound edge was measured during the next 6 h at 2 h intervals by measuring the change in cell free area. The rate of migration was calculated from the change of cell free area measured at 0 and 6 h, as described previously[18].
The results are expressed as mean ± 95% confidence intervals. Student’s unpaired t-test was used for statistical analysis of the raw data. P values less than 0.05 were considered as statistically significant.
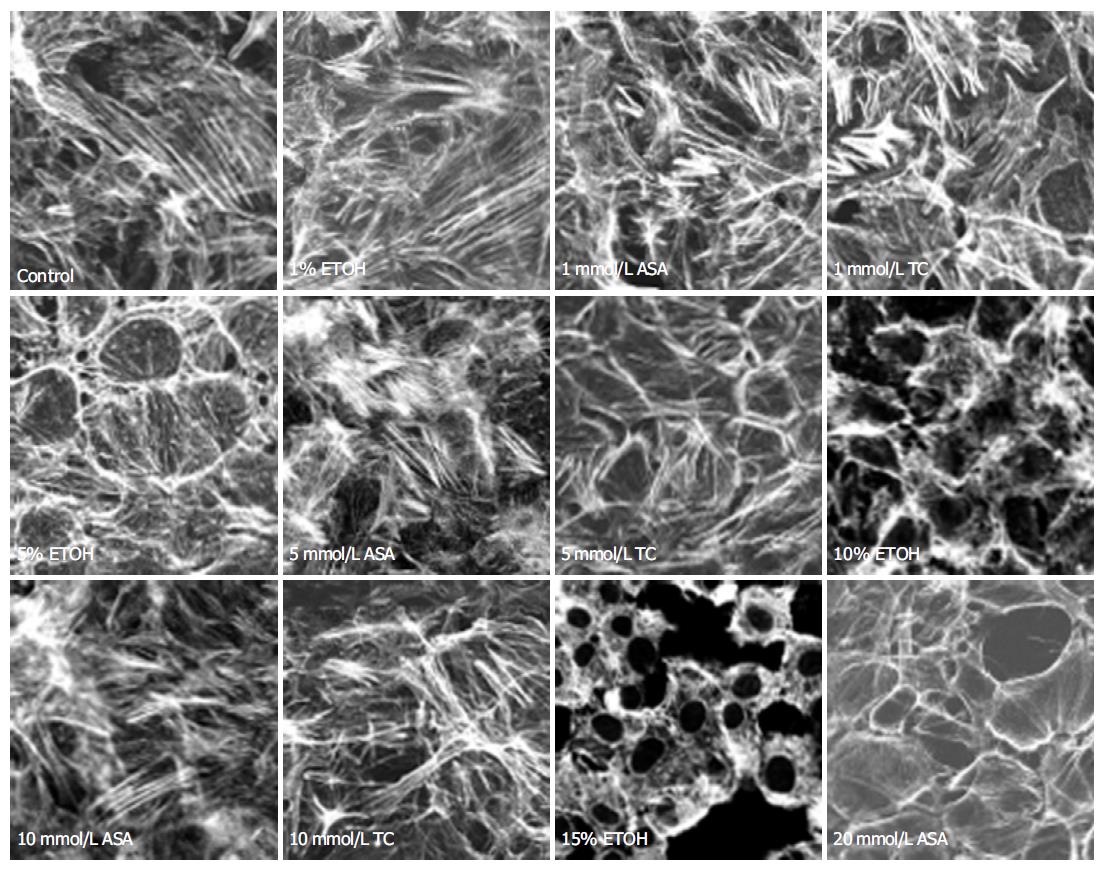
In control cells, actin filaments were thin, showing branched actin networks and zonula adherens-associated actin bundles near the cell-cell contact sites (Figures 1 and 2). Exposure to 10 mL/L (vol/vol) ethanol for 15 min (Figure 1) did not cause any apparent changes in actin cytoskeleton. Increasing the ethanol concentration to 50 mL/L caused moderate degradation and irregularity in the actin filament organization. These changes became more prominent with 100 mL/L ethanol, displaying almost complete degradation of the actin filaments. Exposure to 150 mL/L ethanol for 15 min provoked detachment of the cells from each other (Figure 1). The damage of the actin filaments was severe, and in some microscopic fields only shadows of cells and nuclei remained visible.
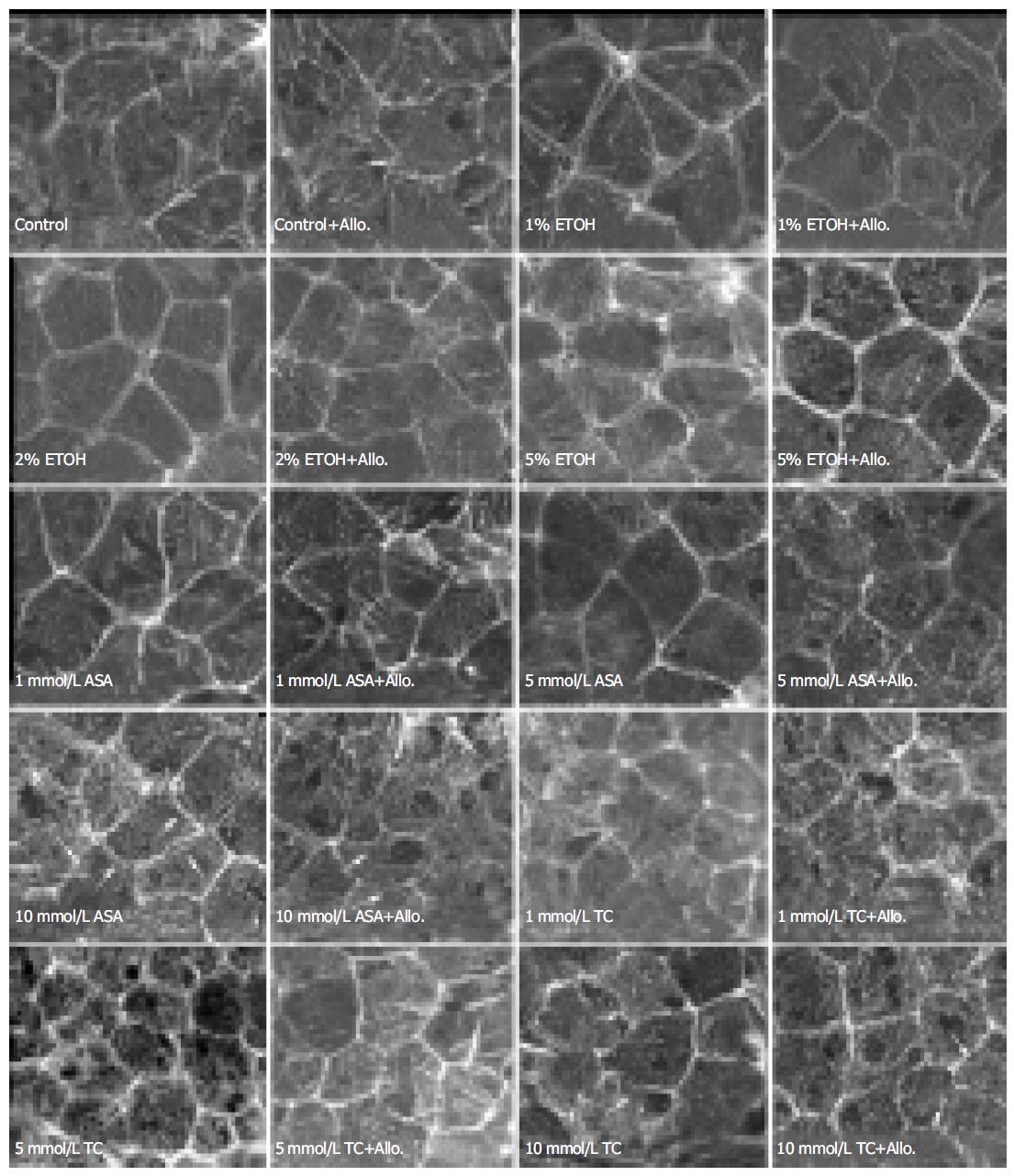
In control cultures, the zonula adherens-associated actin bundles were normally seen as continuous belt-like accumu-lations between the cells, and the actin belts of adjacent cells could not be distinguished from each other. Exposure to 10 mL/L ethanol caused actin belts of adjacent cells to diverge from each other and the gap between them was widened with increasing ethanol concentrations (20-50 mL/L, Figure 2). Treatment with allopurinol (2 mmol/L), which acts as an inhibitor of xanthine oxidase and inhibits production of intracellular reactive oxidative species, prevented completely the divergence of actin belts in cells exposed to 10 mL/L ethanol and partially in cells exposed to 20 mL/L ethanol, but not in cells exposed to 50 mL/L ethanol.
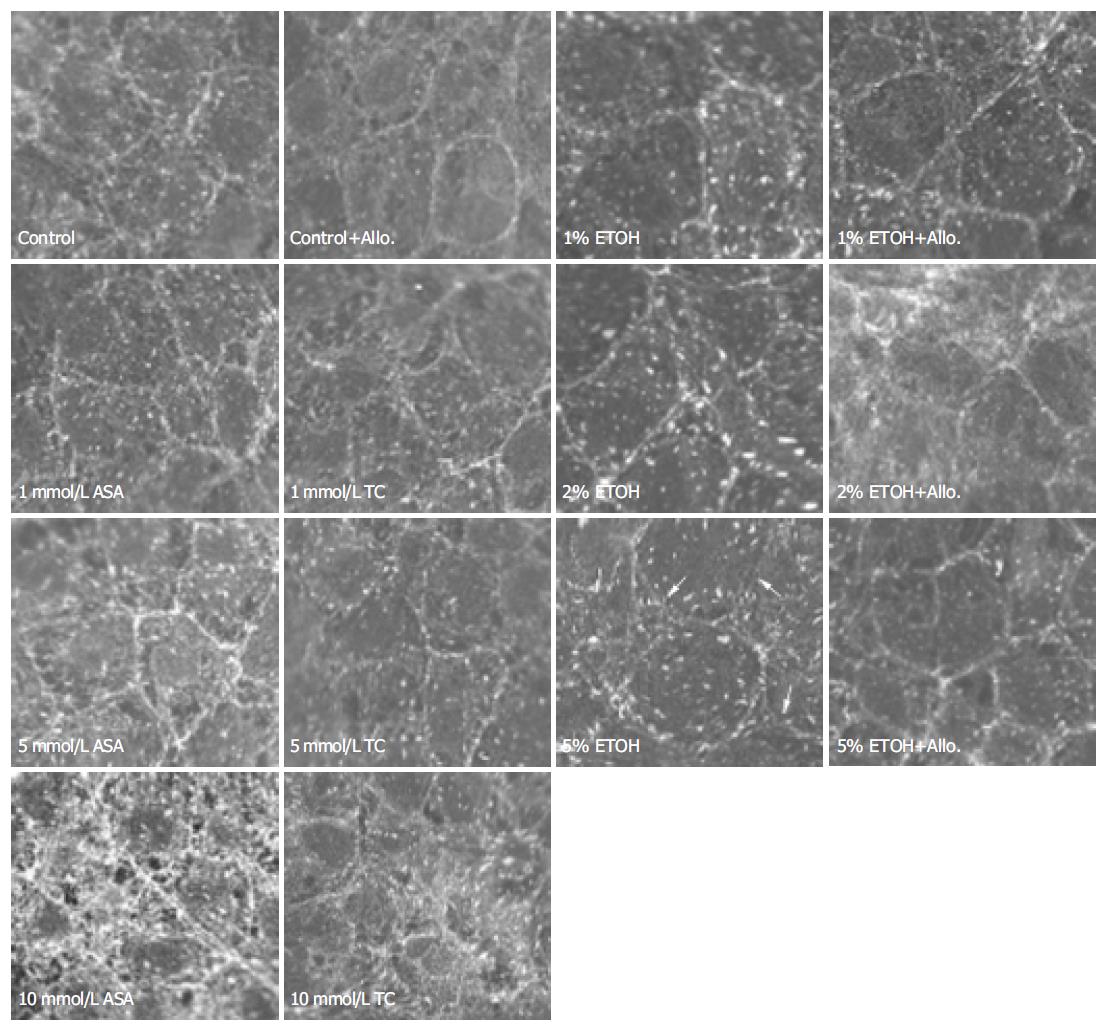
In control cultures, vinculin was visible at the focal adhesions as well as in the zonula adherens between the adjacent cells. The zonula adherens-associated vinculin of adjacent cells could not be distinguished. Exposure to 10-20 mL/L ethanol did not change the distribution of vinculin, but 50 mL/L ethanol exposure caused degradation and divergence of zonula adherens-associated vinculin staining. Allopurinol treatment moderately attenuated this change (Figure 3).
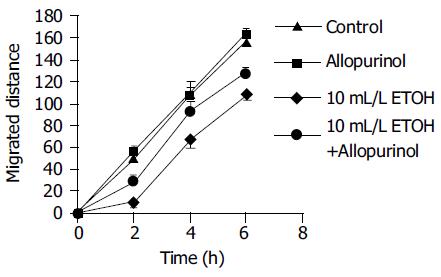
Exposure to 10 mL/L ethanol decreased the rate of migration during the first six hours after wounding (27 ± 3 and 17 ± 2 µm/h for control and ethanol experiments, respectively; P < 0.001, n = 20, Figure 4). This decrease in migration was partially abolished by allopurinol pretreatment (17 ± 2 and 23 ± 2 µm/h for ethanol and ethanol+allopurinol experiments, respectively; P = 0.025, n = 20). Allopurinol treatment alone had no effect on the rate of migration (27 ± 3 and 27 ± 2 µm/h for control and allopurinol experiments, respectively). The migration distances are shown in Figure 4. With higher ethanol concentrations the rate of migration was further decreased (to 7 ± 3 µm/h with 30 mL/L ethanol) and even numerically reversed (to -17 ± 13 µm/h with 50 mL/L ethanol), indicating enlarging of the wound, possibly due to a direct toxic effect of ethanol and/or to overall cell shrinkage in the monolayer caused by ethanol. At these higher concentrations of ethanol (30-50 mL/L) allopurinol did not prevent the ethanol promoted decrease in migration.
After 15 min exposure to 1 mmol/L ASA, the actin filaments were contracted and became shorter and thicker, but no signs of cell detachment from each other were visible. However, with higher concentrations of ASA (10 and 20 mmol/L) the microfilaments became thinner and longer again, but failed to resume their original size and shape. Increasing ASA concentration to 20 mmol/L detached the cells partially from each other, which was manifested also as a divergence of the zonula adherens-associated actin bundles of adjacent cells (Figure 1). Also degradation of the actin filaments was visible.
Exposure to 1-5 mmol/L ASA had no effect on the zonula adherens-associated belt-like actin bundles. Exposure to 10 mmol/L ASA moderately distorted these actin belts and this was not prevented with allopurinol treatment (Figure 2). Exposure to 1-10 mmol/L ASA had no effect on vinculin distribution (Figure 3). Likewise, exposure to 1-5 mmol/L ASA had no effect on the rate of migration, but exposure to 10 mmol/L ASA retarded migration after wounding from 16.8 ± 0.5 to 13.7 ± 1.2 µm/h ( P = 0.008, n = 6).
Following exposure to low concentrations of taurocholate (1 and 5 mmol/L) the actin filaments were contracted, becoming shorter and thicker than in controls. These effects became more conspicuous with increasing concentrations of taurocholate (Figure 1). In fact, taurocholate caused similar changes as was seen with low concentrations of ASA, but the alterations were of lesser degree. Exposure to 1-5 mmol/L taurocholate did not cause any change in the zonula adherens-associated actin bundles, but 10 mmol/L taurocholate showed a moderate perturbation of these belts, which was not prevented by allopurinol treatment (Figure 2). Exposure to taurocholate (1-10 mmol/L) had no effect on vinculin distribution (Figure 3).
Exposure to 1 mmol/L taurocholate had no effect on the rate of migration after wounding, but 5-20 mmol/L taurocholate retarded migration dose dependently (migration speed 16.8 ± 0.6, 14.5 ± 0.8 (P = 0.01), 12.7 ± 0.4 (P < 0.001) and 1.6 ± 0.8 (P < 0.001) µm/h for 0 mmol/L (control), 5, 10 and 20 mmol/L taurocholate, respectively; P values are given in comparison with control, n = 6).
The gastric mucosa is normally exposed to a large number of ulcerogenic compounds, including ethanol, ASA (aspirin) and bile salts. The effects of these agents on cell cytoskeleton have not been thoroughly studied. In the healing of superficial gastric mucosal lesions, intact actin cytoskeleton is essential for cell migration and survival. The normal wound healing process in gastric mucosa is initiated by epithelial restitution whereby the surviving epithelial cells around wound edge migrate over the injured area to cover it with a flattened neo-epithelium. The migrating cells form lamellipodia and vinculin, RhoA and Rac are strongly expressed along the wound edge[19]. Ulcerogenic agents can profoundly modulate this healing process.
Ethanol promotes narrowing of lamellipodia as well as retards cell migration and inhibits cellular proliferation after wounding in rabbit gastric mucosa[20,21]. Exposure to 50-100 mL/L ethanol causes extensive disorganization and fragmentation of the actin cytoskeleton leading to its complete collapse in an intestinal epithelial cell line[22,23]. We have previously shown that 50 mL/L luminal ethanol causes the opening of basolateral potassium channels leading to cell volume shrinkage[24]. In the present work we demonstrated that the same concentration of ethanol affects also various components of the cytoskeleton. The changes in the structure and arrangement of the cytoskeleton are in line with cell shrinkage and the consequent increase in the gaps between the cells. Since an intact cytoskeleton is of utmost importance for the epithelial integrity and function, these minor changes may be the first manifestations of the ethanol-induced damage to the epithelial cells. At 100 mL/L ethanol concentration, there was an extensive distortion of the actin cytoskeletal network, and even stronger ethanol (150 mL/L) caused severe damage to the cells with complete degradation of all actin filaments.
The zonula adherens-associated belt-like actin bundles of adjacent cells were diverged from each other already during exposure to a rather low (10 mL/L) concentration of ethanol. This divergence was prevented with 2 mmol/L allopurinol treatment. Allopurinol is a cell membrane permeable xanthine oxidase inhibitor, which blocks endogenous intracellular enzymatic generation of superoxide radicals from purines. This suggests that superoxide radicals are involved in ethanol induced divergence of the actin belts. Ethanol also perturbed the distribution of the zonula adherens junction-associated protein, vinculin, but only after exposure to 50 mL/L ethanol. Further, this change was moderately opposed by allopurinol treatment. The damage in zonula adherens-associated vinculin became apparent only with higher ethanol concentrations than was needed for changes in zonula adherens-associated actin bundles, which suggests that the changes in the actin belts might precede the zonula adherens junction damage. Allopurinol also opposed the ethanol provoked decrease in cell migration after artificial monolayer wounding, a further indication of the involvement of superoxide radicals in ethanol induced cytoskeletal damage.
The above findings suggest that oxidative stress might underlie the observed ethanol induced changes in actin cytoskeleton. Epithelial cells exposed to ethanol increase superoxide production[25] and superoxide dismutase activity is decreased in rat stomach exposed to pure ethanol[26]. Also, oxidative stress and mitochondrial damage precede death in gastric mucosal cells exposed to ethanol[15]. The number of propidium iodide positive cells, indicating loss of cell viability, was increased following exposure to 50 mL/L ethanol and ethanol caused mitochondrial cell membrane depolarization and mitochondrial permeability transition, indicating mitochondrial dysfunction[15].
It has been suggested, that NSAIDs, including ASA, damage gastrointestinal tract both by direct local effects and by systemic inhibition of prostaglandin synthesis. Davenport first demonstrated that ASA diffuses into the gastric mucosa as an undissociated molecule leading to disruption of the gastric mucosal barrier and backdiffusion of luminal acid into the mucosa, which, in turn, leads, presumably via generation of an inflammatory cascade, to break-down of the mucosal tissue[27]. Although prostaglandins contribute to cell migration in several cell types[28,29], addition of exogenous prostaglandins or inhibition of endogenous prostaglandin synthesis had no effect on gastric epithelial cell migration[18]. On the other hand, NSAIDs may directly retard cell migration and decrease actin staining in gastric epithelial cell (RGM1) monolayer[30].
The present results with ASA are well in accordance with our earlier finding that ASA causes major cell shape deformations in Necturus gastric antrum (unpublished results). The shrinkage of actin cytoskeleton probably contributes to cell-cell detachment, which was shown to occur at higher ASA concentrations. ASA decreased migration at 10 mmol/L concentration and degradation of actin filaments was visible at 20 mmol/L ASA concentration. Zonula adherens-associated actin bundles were moderately damaged with 10 mmol/L ASA and this was not affected by allopurinol treatment, suggesting a mechanism different from ethanol-induced divergence of these actin bundles.
To our knowledge there is very little information about the actions of ASA on cellular actin cytoskeleton in the gastric epithelium. Electrophysiological measurements have shown that there is a slight initial increase in transepithelial resistance following exposure to 10 mmol/L ASA (at pH 3), which is, however, due to increase in apical cell membrane resistance. However, during more prolonged exposure to ASA the transepithelial resistance decreased, which might be a consequence of tight junction disruption, widening of intercellular space and/or loosening of cell-cell adhesions[31]. The changes in the zonula adherens-associated actin bundles observed in the present study might contribute to the previously observed decrease in transepithelial resistance.
In earlier studies bile salts have been shown to retard wound restitution and repair in cultured rabbit gastric epithelial cells[32]. In the present study, taurocholate caused similar effects in cell migration reducing it dose dependently in concentrations of 5 mmol/L and higher. The mechanism of taurocholate induced decrease in migration speed is still unclear, but bile salts are known to increase intracellular calcium levels[33,34] and this might have an effect on cellular migration.
Taurocholate is known to interact with cell membranes and as a lipophilic compound it may diffuse into the cell mem-branes increasing its permeability to extracellular agents[35]. Taurocholate may also act as a detergent dissolving cell membrane phospholipids eventually breaking up the cell membrane[36]. Apical cell membrane resistance was decreased significantly with 10 mmol/L taurocholate in Necturus antrum[31], suggesting that cell membranes may be the main target in taurocholate induced cellular damage. Zonula adherens associated actin bundles were moderately damaged with 10 mmol/L taurocholate, but this was not affected by allopurinol treatment suggesting, again, a mechanism different from ethanol induced damage. Previously, it has been shown that bile salts induce depolarization of mitochondrial membrane potential, which might be due to disturbed oxidative phosphorylation in mitochondria[37]. In the present study, the possible oxidative damage was not prevented with allopurinol exposure, suggesting that xanthine oxidase is not involved in taurocholate-induced cellular injury.
In conclusion, our results show that deformations of the actin cytoskeleton are involved in the epithelial cell damage caused by the three ulcerogenic agents tested. Reactive oxidative species seems to underlie ethanol, but not ASA or taurocholate, induced cytoskeletal damage.
The authors thank Ms. Paula Kokko for excellent technical assistance.
Co-first-authors: Harri Mustonen
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Luby-Phelps K. Physical properties of cytoplasm. Curr Opin Cell Biol. 1994;6:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 146] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Seksek O, Biwersi J, Verkman AS. Translational diffusion of macromolecule-sized solutes in cytoplasm and nucleus. J Cell Biol. 1997;138:131-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 417] [Cited by in RCA: 382] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 3. | Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th ed. New York: Garland Science 2002; . |
| 4. | Ayscough KR. In vivo functions of actin-binding proteins. Curr Opin Cell Biol. 1998;10:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 126] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Ku NO, Zhou X, Toivola DM, Omary MB. The cytoskeleton of digestive epithelia in health and disease. Am J Physiol. 1999;277:G1108-G1137. [PubMed] |
| 6. | Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1204] [Cited by in RCA: 1167] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 7. | Weber I, Gerisch G, Heizer C, Murphy J, Badelt K, Stock A, Schwartz JM, Faix J. Cytokinesis mediated through the recruitment of cortexillins into the cleavage furrow. EMBO J. 1999;18:586-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114:2713-2722. [PubMed] |
| 9. | Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu Rev Biophys Biomol Struct. 2000;29:545-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1160] [Cited by in RCA: 1098] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 10. | Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777-1788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1844] [Cited by in RCA: 1874] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 11. | Critchley DR. Focal adhesions - the cytoskeletal connection. Curr Opin Cell Biol. 2000;12:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 425] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 12. | Pokutta S, Weis WI. The cytoplasmic face of cell contact sites. Curr Opin Struct Biol. 2002;12:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | DeMali KA, Barlow CA, Burridge K. Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J Cell Biol. 2002;159:881-891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 322] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 14. | Higuchi H, Adachi M, Miura S, Gores GJ, Ishii H. The mitochondrial permeability transition contributes to acute ethanol-induced apoptosis in rat hepatocytes. Hepatology. 2001;34:320-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Hirokawa M, Miura S, Yoshida H, Kurose I, Shigematsu T, Hokari R, Higuchi H, Watanabe N, Yokoyama Y, Kimura H. Oxidative stress and mitochondrial damage precedes gastric mucosal cell death induced by ethanol administration. Alcohol Clin Exp Res. 1998;22:111S-114S. |
| 16. | Bellomo G, Mirabelli F. Oxidative stress and cytoskeletal alterations. Ann N Y Acad Sci. 1992;663:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Kobayashi I, Kawano S, Tsuji S, Matsui H, Nakama A, Sawaoka H, Masuda E, Takei Y, Nagano K, Fusamoto H. RGM1, a cell line derived from normal gastric mucosa of rat. In Vitro Cell Dev Biol Anim. 1996;32:259-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Ranta-Knuuttila T, Kiviluoto T, Mustonen H, Puolakkainen P, Watanabe S, Sato N, Kivilaakso E. Migration of primary cultured rabbit gastric epithelial cells requires intact protein kinase C and Ca2+/calmodulin activity. Dig Dis Sci. 2002;47:1008-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Watanabe S, Hirose M, Yasuda T, Miyazaki A, Sato N. Role of actin and calmodulin in migration and proliferation of rabbit gastric mucosal cells in culture. J Gastroenterol Hepatol. 1994;9:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Murai T, Watanabe S, Hirose M, Miwa H, Miyazaki A, Sato N. Ethanol retards gastric epithelial restoration in monolayer cultures. Dig Dis Sci. 1996;41:2062-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Murai T, Watanabe S, Hirose M, Kobayashi O, Maehiro K, Ohkura R, Miwa H, Kitamura T, Ogihara T, Oide H. Evaluation of ethanol on gastric epithelial restoration in vitro. Alcohol Clin Exp Res. 1996;20:45A-46A. [PubMed] |
| 22. | Banan A, Smith GS, Kokoska ER, Miller TA. Role of actin cytoskeleton in prostaglandin-induced protection against ethanol in an intestinal epithelial cell line. J Surg Res. 2000;88:104-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Miller TA, Smith GS, Banan A, Kokoska ER. Cytoskeleton as a target for injury in damaged intestinal epithelium. Microsc Res Tech. 2000;51:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Mustonen H, Kivilaakso E. Effect of luminal ethanol on epithelial resistances and cell volume in isolated Necturus gastric mucosa. Dig Dis Sci. 2003;48:2037-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Hiraishi H, Shimada T, Ivey KJ, Terano A. Role of antioxidant defenses against ethanol-induced damage in cultured rat gastric epithelial cells. J Pharmacol Exp Ther. 1999;289:103-109. [PubMed] |
| 26. | Kwiecień S, Brzozowski T, Konturek SJ. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J Physiol Pharmacol. 2002;53:39-50. [PubMed] |
| 27. | Davenport HW. Gastric mucosal injury by fatty and acetylsalicylic acids. Gastroenterology. 1964;46:245-253. [PubMed] |
| 28. | Joyce NC, Meklir B. PGE2: a mediator of corneal endothelial wound repair in vitro. Am J Physiol. 1994;266:C269-C275. [PubMed] |
| 29. | Gotlieb AI. Prostaglandin induced shape changes in fibroblasts grown in cell culture. Prostaglandins. 1980;19:865-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Pai R, Szabo IL, Giap AQ, Kawanaka H, Tarnawski AS. Nonsteroidal anti-inflammatory drugs inhibit re-epithelialization of wounded gastric monolayers by interfering with actin, Src, FAK, and tensin signaling. Life Sci. 2001;69:3055-3071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Kiviluoto T, Mustonen H, Kivilaakso E. Effect of barrier-breaking agents on intracellular pH and epithelial membrane resistances: studies in isolated Necturus antral mucosa exposed to luminal acid. Gastroenterology. 1989;96:1410-1418. [PubMed] |
| 32. | Watanabe S, Wang XE, Hirose M, Yoshizawa T, Iwazaki R, Oide H, Kitamura T, Miwa H, Miyazaki A, Sato N. Effects of rebamipide on bile acid-induced inhibition of gastric epithelial repair in a rabbit cell culture model. Aliment Pharmacol Ther. 1996;10:927-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Dziki AJ, Batzri S, Harmon JW, Molloy M. Cellular hypercalcemia is an early event in deoxycholate injury of rabbit gastric mucosal cells. Am J Physiol. 1995;269:G287-G296. [PubMed] |
| 34. | Molloy M, Batzri S, Dziki AJ, Goldberg WJ, Hale DA, Harmon JW. Reversibility of deoxycholate-induced cellular hypercalcemia in rabbit gastric mucosal cells. Surgery. 1996;119:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Duane WC, Wiegand DM, Sievert CE. Bile acid and bile salt disrupt gastric mucosal barrier in the dog by different mechanisms. Am J Physiol. 1982;242:G95-G99. [PubMed] |
| 36. | Thomas AJ, Nahrwold DL, Rose RC. Detergent action of sodium taurocholate on rat gastric mucosa. Biochim Biophys Acta. 1972;282:210-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Miura S, Fukumura D, Shiozaki H, Suzuki M, Kurose I, Suematsu M, Tsuchiya M, Ishii H. Bile acid-induced depolar-ization of mitochondrial membrane potential preceding cell injury in cultured gastric mucosal cells. J Gastroenterol Hepatol. 1995;10:621-626. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |