Published online Jul 14, 2005. doi: 10.3748/wjg.v11.i26.4024
Revised: October 3, 2004
Accepted: October 7, 2004
Published online: July 14, 2005
AIM: Eph receptor tyrosine kinases and their membrane bound receptor-like ligands, the ephrins, represent a bi-directional cell-cell contact signaling system that directs epithelial movements in development. The meaning of this system in the adult human gut is unknown. We investigated the Eph/ephrin mRNA expression in the intestinal epithelium of healthy controls and patients with inflammatory bowel disease (IBD).
METHODS: mRNA expression profiles of all Eph/ephrin family members in normal small intestine and colon were established by real-time RT-PCR. In addition, differential expression in IBD was investigated by cDNA array technology, and validated by both real-time RT-PCR and immunohistochemistry. Potential effects of enhanced EphB/ephrin-B signaling were analyzed in an in vitro IEC-6 cell scratch wound model.
RESULTS: Human adult intestinal mucosa exhibits a complex pattern of Eph receptors and ephrins. Beside the known prominent co-expression of EphA2 and ephrinA1, we found abundantly co-expressed EphB2 and ephrin-B1/2. Interestingly, cDNA array data, validated by real-time PCR and immunohistochemistry, showed upregulation of ephrin-B2 in both perilesional and lesional intestinal epithelial cells of IBD patients, suggesting a role in epithelial homeostasis. Stimulation of ephrin-B signaling in ephrin-B1/2 expressing rat IEC-6-cells with recombinant EphB1-Fc resulted in a significant dose-dependent acceleration of wound closure. Furthermore, fluorescence microscopy showed that EphB1-Fc induced coordinated migration of wound edge cells is associated with enhanced formation of lamellipodial protrusions into the wound, increased actin stress fiber assembly and production of laminin at the wound edge.
CONCLUSION: EphB/ephrin-B signaling might represent a novel protective mechanism that promotes intestinal epithelial wound healing, with potential impact on epithelial restitution in IBD.
- Citation: Hafner C, Meyer S, Langmann T, Schmitz G, Bataille F, Hagen I, Becker B, Roesch A, Rogler G, Landthaler M, Vogt T. Ephrin-B2 is differentially expressed in the intestinal epithelium in Crohn’s disease and contributes to accelerated epithelial wound healing in vitro. World J Gastroenterol 2005; 11(26): 4024-4031
- URL: https://www.wjgnet.com/1007-9327/full/v11/i26/4024.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i26.4024
The rapid resealing of the intestinal surface partly relies on the highly adaptive ability of epithelial wound-edge cells to rapidly form pseudopodial protrusions, reorganize the cytoskeleton, and migrate into a wound defect in a coordinated manner[1-3]. In the intestine, the fast epithelial healing is particularly important considering the necessity to protect the host with a one-cell-layer epithelium against a considerable microbial threat and exposure to a multitude of immunogenic and toxic factors[1]. Since epithelial integrity is critically linked to the prevention and healing of inflammatory bowel disease (IBD), the investigation of wound closure modifying factors has attracted a lot of attention in this field. Growth factors (EGF, TGF-β, HGF, KGF, IGF), trefoil peptides, cytokines (IL-1β, IL2), nutrients (SCFA, polyamines), and matrix components (laminin) are among the best studied contributors to epithelial restitution[1,4].
In contrast to those established soluble co-factors, the Eph receptor tyrosine kinases (RTKs), and their membrane-bound receptor-like ligands, the ephrins, specifically direct collaborative cell movements by direct cell-cell signaling[5,6]. Based on their sequence homology, structure, and binding affinity, the Eph-RTKs and ephrins are divided into two subclasses, A and B. With few exceptions, the two classes (A and B) of Eph-RTKs exhibit a differential affinity for the distinct A- and B-subsets of ephrins. In contrast to humoral signaling systems, the ligands in this system are membrane-bound. A-ephrins are tethered to the outer leaflet of the plasma membrane by virtue of a glycosyl-phosphatidylinositol (GPI) anchor, whereas, B-ephrins are transmembrane proteins[7,8]. With the discovery that ephrins can by themselves induce a “reverse” cellular signaling response by exhibiting receptor-like functions, it is apparent that the Eph-RTK/ephrin interactions represent a cell-cell signaling system that is capable of mediating a bidirectional response in both the ligand and the receptor-bearing cell[9]. The generated juxtacrine signals organize the coordinated movement of cells during development, e.g. in axonal guidance, remodelling of blood vessels, and formation of epithelial surfaces[10-13]. It has also been demonstrated that the differential expression of ephrin ligands and Eph-RTKs along the crypt-villus axis determines the correct positioning and allocation of the proliferating versus differentiating compartment during development of crypts in the small intestine[14]. Disruption of those genes leads to intermingling between both of these compartments.
While the meaning of Eph-RTKs/ephrins in embryonic morphogenesis has been well established, their physiologic role in adult tissues is unknown. However, recent data indicate a possible role in tissue repair and maintenance[15]. EphA2/ephrin-A1 signaling, previously termed epithelial cell kinase (Eck) and protein B61, has already been suggested to be involved in the homeostasis of the intestinal barrier in adults[16]. Since cytokines such as IL-1β, IL-2 and TGF-β can modulate the steady-state levels of the expression of EphA2/ephrin-A1 in vitro, a role in inflammatory bowel disease (IBD) is possible[16]. Therefore, we investigated the full repertoire of Eph-RTKs and ephrins in the gut epithelium and possible differential expression in IBDs. Furthermore, possible consequences of the observed changes for the intestinal wound closure capacity were evaluated.
The corresponding protocols for the study have been accepted by the local ethics committee and biopsies were taken after informed consent of the patients. For establishing of an Eph-RTK/ephrin mRNA expression profile of the normal human gut, total mucosa biopsies were obtained from normal colon (n = 5, ages ranged from 28 to 75 years). Normal small intestine mRNAs (n = 5, ages ranged from 20 to 61 years) were purchased from Clontech (BD Biosciences, Palo Alto, USA). For each tissue, the RNAs were pooled to avoid bias due to potential interindividual differences.
Affymetrix® HGU133A and HGU133B GeneChip array data, recently published by our collaborators, indicated possible differential expression of some Eph-RTK/ephrin family members[17]. In that array analysis, samples had been collected from resected terminal ileum and colon of control subjects (n = 4, ages ranged from 52 to 60 years), Morbus Crohn (MC) patients (n = 4, ages ranged from 23 to 43 years), and Colitis ulcerosa (CU) patients (n = 4, ages ranged from 20 to 57 years). The material was obtained from non-inflamed regions at least 10 cm distant from visibly inflamed areas to avoid bias due to inflammatory cells. From the same cohort of patients, individual (not pooled) RNAs were available for real-time RT-PCR based validation of selected genes in this study.
For low density array analysis, biopsies were used provided by the core facility “primary cells and tissues” of the Regensburg-SFB585 (German interdisciplinary research initiative on “immune functions in the gut”; http://www. uniregensburg.de/Einrichtungen/Klinikum/SFB). To control the sampling process, sets of six biopsies were taken from each patient or control subjects from identical sites. One of those biopsies was archived and paraffin-embedded for histopathological analysis by an expert pathologist (F.B.). The remaining biopsies were used for research purposes in this set of experiments. Bias due to stromal and inflammatory cells was avoided by preparation of intestinal epithelial cells (IECs). In brief, five needle-head sized biopsies were sampled in 10 mL HBSS/2 mmol/L EDTA buffer. Epithelial cells and crypts were detached by vigorous shaking at 37°C for 15 min at 225 r/min. After vortexing for a few seconds, the remaining parts of the biopsies were manually removed. Crypts and cells were then spun down by short centrifugation and resolved directly into 350 µL RLT buffer (RNeasy, Qiagen, Hildesheim, Germany). Due to this IEC preparation, IBD biopsies could be investigated from actively inflamed sites in this series. Four control samples (ages ranged from 22 to 66 years), seven MC samples (ages ranged from 33 to 48 years) and four CU samples (ages ranged from 28 to 62 years) were included.
For all samples, RNA extraction was performed according to the manufacturer’s protocol (RNeasy Mini Kit, Qiagen, Hilden, Germany). RNA quality was assessed with the 6000 Nano LabChip® using an Agilent 2100 bioanalyzer (Agilent Technologies, Palo Alto, USA) and quantified spectrophoto-metrically following standard protocols.
Real-time TaqMan® RT-PCR (PE Applied Biosystems, Darmstadt, Germany) was performed on a ABI Prism 7900 HT Sequence Detection System as previously published[15,18]. Briefly, cDNA was synthesized using the Reverse Transcription Kit from Promega (Madison, USA) according to the manufacturer’s protocol. Probes and primers for TaqMan® analysis were designed on the basis of gene-specific nonhomologous DNA sequence of the corresponding members[15]. The standard curve method was used for the quantification of the relative amounts of gene expression products. This method provides unitless normalized expression values that can be used for direct comparison of the relative amounts of target mRNA in different samples. All reactions were performed as triplicates.
Due to limited representation of the Eph-RTK/ephrin family on the Affymetrix® HGU 133 A/B chips, we established a custom low density array comprising 91 important RTKs, including the complete family of Eph-RTKs/ephrins and a selection of other receptor and non-receptor tyrosine kinases. The selection of non-Eph-RTKs was based on a current review on kinase signaling[19]. The detailed list of genes can be requested from the authors.
The low density arrays were prepared as follows. 50-mer amino modified oligonucleotide probes were arrayed onto CodeLink™ Slides (Amersham Biosciences) using a conventional Pin-and-Ring™ spotter (Affymetrix) in a controlled environment (20°C and 65-75% humidity). Optimal sequences of the 50mer probes (two for each gene) were determined by a MWG Biotech proprietary software (MWG Biotech, Ebersberg, Germany). The 2 × 91 oligonucleotides were spotted together with 9 reference oligos (mwghuman10K #11000-#11008) in linear pattern with 4 replicates each, resulting in a total number of 955 spots per slide. Labeled cRNA was synthesized from total RNA using Cy3-UTP (Amersham Biosciences) in accordance with the linear RNA amplification procedures described in the MWG Array-Application Guide. The cRNA was applied onto the microarray surface using 120 µL Gene Frames® (ABgene) and hybridized for 16 h at 42°C. For data analysis, the microarrays were scanned at 10 micron resolution and analyzed using the program ScanAlyze (M. Eisen; available at http://rana.lbl.gov/EisenSoftware.htm). Areas of the array with obvious bad signals were manually flagged and excluded from subsequent analyses. A linear normalization method was used, assuming that most of the genes represented on an array show the same expression profile. Expression ratios were then calculated and further evaluated using the Significance Analysis of Microarrays (SAM) algorithm[20]. The SAM calculates the false discovery rate (FDR) as an estimate of the percentage of false positive genes, i.e. genes identified as differentially expressed by chance. Two-class unpaired analysis was applied to compare MC and CU samples to the control samples, separately. Identical FDRs were used to compare the significance of the regulated genes in MC vs control and CU vs control expression patterns.
For immunohistochemical staining of ephrin-B2 the anti-ephrin-B2 antibody (Santa Cruz, CA, USA) was used in a dilution of 1:75. Sections of intestinal tissue were boiled (10 min) in citrate buffer for antigen retrieval. Isotype matched antibody controls were run in parallel. The remaining staining procedure according to the ABC-technique followed standard protocols. Ten control sections of healthy individuals (ages ranged from 43 to 78 years), 10 cases of CU (ages ranged from 26 to 80 years), and 10 cases of MC (ages ranged from 21 to 49 years) were analyzed.
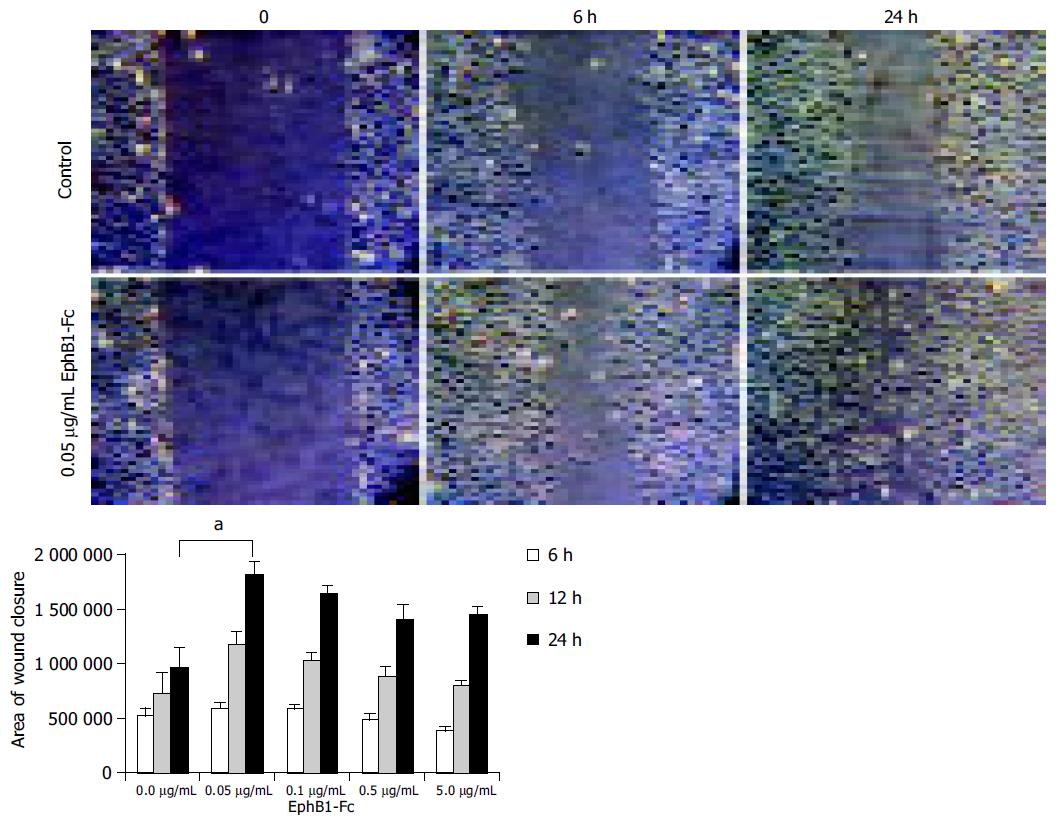
To evaluate possible consequences of enhanced ephrin-B dependent signaling effects on wound closure of intestinal cells, not transformed rat IEC-6 cells were chosen as a model. IEC-6 cells are able to close monolayer scratch wounds within a timeframe assuring that closure is a function of migration competence of the cells at the wound margin rather than proliferation[21]. In our hands, both HT29 cells and CaCo2 cells failed to fulfil this criterion. Briefly, the IEC-6 cells were seeded into six-wells and grown to confluence in Dulbecco’s MEM (Biochrom AG, Berlin, Germany) supplemented with 5% fetal calf serum (FCS) (PAN Biotech, Aldenbach, Germany). After reaching confluence, the cells were fed with starving medium (0.1% FCS) and small scratch-wounds (-1 mm width) were produced with a cell scraper drawn over the surface of the monolayer along a ruler. The cells were stimulated with varying concentrations of recombinant rat EphB1-Fc Chimera (R&D Systems, Minneapolis, USA). The used doses ranged from 0.05 µg/mL (~0.33 nmol/L) to 5 µg/mL (~33 nmol/L). For control experiments, recombinant human IgG-Fc (R&D Systems) was used. Wound closure was monitored at 0, 6, and 24 h after wounding. Images of the wounds were taken by a digital camera on top of the cell culture microscope from exactly the same position at various time points (100 × magnification). The closure of the wounds was assessed by calculating the difference between the initial wound area and the remaining wound area at a certain time point. Wound area calculations were performed using the publicly available J-Image software of the NCBI (Bethesda, MA, USA). For each condition, four wounded areas were analyzed in parallel, and all experiments were repeated twice.
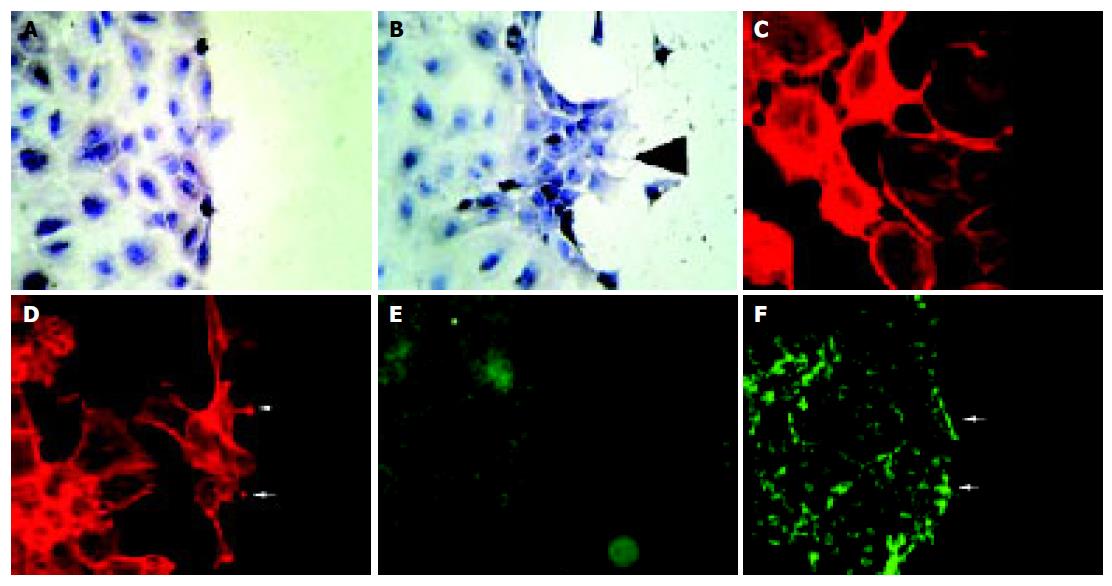
Cells were plated on fibronectin coated glass cover slips and grown for 16 h in Dulbecco’s MEM plus 5% FCS. Cells were starved for one night with 0.1% FCS medium, then the monolayer was wounded by a cell scraper and stimulated with recombinant EphB1-Fc (5 µg/mL) or IgG-Fc (5 µg/mL) as control for 6 h. For actin staining, cells were fixed with 4% paraformaldehyde for 5 min at room temperature and permeabilized with 0.1% Triton-X 100 for 5 min. The cells were then incubated for 30 min with rhodamine-conjugated phalloidine (1:100) (Chemicon, Temecula, CA, USA) as recommended by the supplier. Each experimental condition was performed on eight replicate cover slips. For detection of wound edge deposits of laminin an affinity purified laminin specific antibody L9393 from Sigma (St. Louis, MS, USA) and FITC labeled secondary goat anti-rabbit antibody (Vector, Burlingame, USA) were used. Images were taken on a Leitz microscope equipped with a fluorescence lamp and appropriate filters. Cells were photographed at 400× magnification.
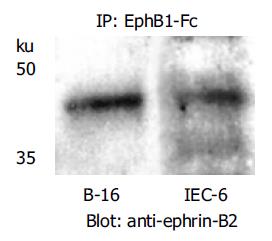
To demonstrate the ability of the recombinant rat EphB1-Fc (R&D Systems) used to bind and potentially activate ephrin-B2 in IEC-6 cells, immunoprecipitation of ephrin-B2 was performed using the SeizeTM Classic Mammalian Immunoprecipitation Kit (Pierce, Rockford, USA). Ephrin-B2 protein was precipitated using this recombinant rat EphB1-Fc. Precipitates were analyzed by SDS-PAGE and blotted according to standard protocols. For detection of ephrin-B2, a polyclonal anti-ephrin-B2 antibody (Santa Cruz) was used.
Non-parametric testing was performed (Mann-Whitney test) for comparing the mean values in the wound closure experiments. Significance was indicated by aP < 0.05.
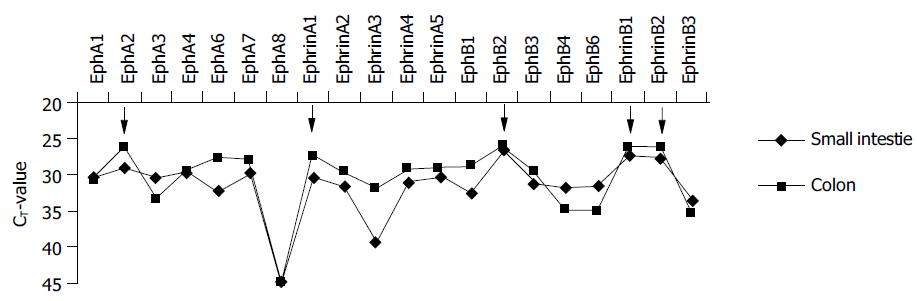
The role of Eph-RTKs and ephrins is well defined in developmental biology. However, the expression and function in adult tissues is only marginally known, yet. Therefore, we established a profile of the expression of the members of this family in the small intestine and colon from normal control individuals. For this purpose, a library of real time TaqMan® RT-PCR probes and primers was employed that was recently established in our lab[15]. The results are shown in Figure 1. Small intestine and colon mucosa exhibit the presence of a broad spectrum of A-and B-class Eph receptors and ephrins with some organotypic differences of the expression profiles. Ephrin-A1 (formerly protein B61) and ephrin-B1/2 are the most abundantly expressed ligands. On the receptor site, EphA2 (formerly epithelial cell kinase, Eck) and EphB2 are the most abundantly expressed receptors.
A recently published gene array analysis (Affymetrix® platform) of pooled IBD samples and control patients produced detectable signals only for a minor fraction of Eph-RTKs and ephrins (EphA2, ephrin-A1, EphB2, EphB4 and ephrin-B1/2)[17]. Interestingly, these were almost exactly the most abundantly expressed genes of this family in our real-time RT-PCR profile (Figure 1).
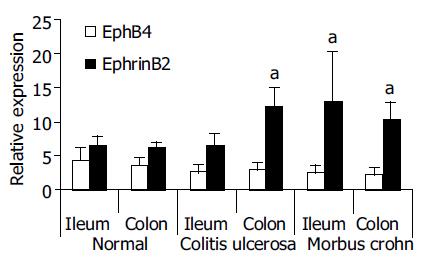
That array analysis had further revealed an up-regulation of ephrin-B2 (2.4-fold in the small intestine and 1.5-fold in the colon) in perilesional, not inflamed mucosa of MC patients[17]. In this study, we validated those observations in the same cohort of patients. For this purpose, we used our established TaqMan® probe and primer set for ephrin-B2. In these experiments, the individual (not pooled) samples of all 4 well characterized cases of MC, CU and controls were analyzed (Figure 2). A significant up-regulation of ephrin-B2 could be confirmed in the individual MC patients both in the small intestine and in the colon samples. The individual probes of the CU patients also showed a significant up-regulation in the colon samples. In contrast, a 0.5-fold downregulation of EphB4 in the colon of CU patients, as indicated by previous Affymetrix® array analysis[17], did not reach significance in the real-time RT-PCR validation.
Since RT-PCR suggested a much more diversified presence of Eph-RTKs and ephrins in gut mucosa than it was detectable by the HGU 133 A/B chip generation, we employed low density 50 mer oligonucleotide technology based on protocols developed by MWG (MWG Biotech, Ebersberg, Germany). To further substantiate the specificity of the results for the epithelial compartment, in this series of experiments the IECs were isolated from sets of biopsies of controls and lesional inflamed mucosa biopsies of MC patients and CU patients, respectively.
SAM (significance analysis of microarray data) analysis revealed that ephrin-B2 reached the highest significance score in MC patients among 16 further genes with significant scores at an estimated false positive rate of 5%. This is consistent with the previous Affymetrix® array analysis[17] and our real time RT-PCR data (Figure 2). Interestingly, with this 50 mer low density chip further Eph-RTKs and ephrins produced measurable signals and were identified as potentially regulated in the (lesional) intestinal epithelium of MC patients (ephrin-A5, EphB1, EphB4). Moreover, FGFR-3, INSRR, NGFR, PDGFR-β were among the candidate genes, growth factor receptors that have already been linked to epithelial healing, proliferation and many other aspects of IBD-physiology[22]. In contrast, no significantly regulated genes were observed in the CU group.
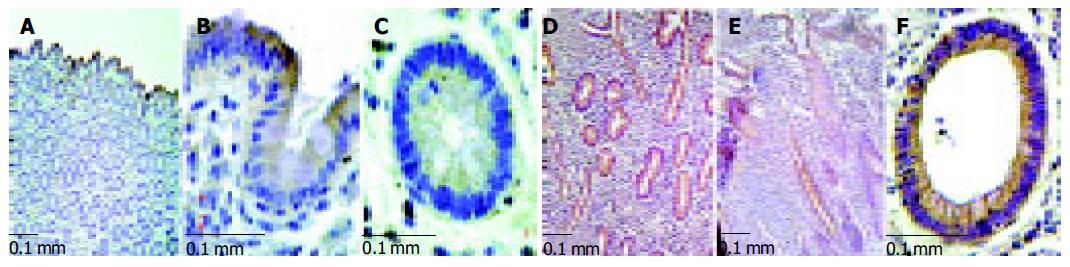
Immunohistochemistry revealed that ephrin-B2 is expressed most prominently in the upper parts of the crypts in normal mucosa of the human colon, i.e. the most intense expression is found in the terminally differentiated epithelium at the luminal surface. Apparently, there is a gradient along the crypt-axis with decreasing expression in the deeper parts of the crypts. In contrast, in inflamed MC samples a strong staining of the entire epithelium was observed from the basis to the top of the crypts with an obvious loss of this gradient (Figure 3). In CU samples, this distortion of ephrin-B2 expression was also seen in some samples, but not as consistent as in MC.
Since both array analysis and quantitative RT-PCR data suggested up-regulation of ephrin-B2 in lesional and perilesional intestinal epithelium of MC patients, we investigated possible functional consequences of intensified reverse ephrin-B signaling in an intestinal epithelial model cell line. Not transformed rat intestinal IEC-6 cells were chosen for two reasons: (1) Constitutive expression of the ligand ephrin-B2 and the receptor EphB2 in this cell line could be confirmed by quantitative RT-PCR, immunocytochemistry (data not shown) and immunoblotting (Figure 4); (2) IEC-6 cells exhibit a basal wound closure competence in vitro, which exceeded the competence of human intestinal cancer cell lines (HT-29, CaCo-2, SW-480) tested in our lab.
Wounded monolayers of IEC-6 cells were treated with recombinant rat EphB1-Fc for stimulation of ephrin-B dependent pathways. As shown in Figure 5, a dose-dependent acceleration of wound closure by stimulation with EphB1 could be observed after 6 and 24 h (P < 0.05). Interestingly, maximal effects were already obtained with 0.05 µg/mL (~0.33 nmol/L) concentration of EphB1-Fc. Higher concentrations slightly decreased the wound closure activity. Control experiments were performed with equal amounts of IgG-Fc.
As shown in Figure 6, starved unstimulated IEC-6 cells exhibit only a limited wound closure activity 6 h after wounding (Figure 6A). In contrast, the EphB1-Fc stimulated cells already show signs of organized motile activity forming multiple archipelago-like sheets of cells stretching into the denuded area (Figure 6B). Notably, asymmetric lamellipodial protrusions are formed by those wound-edge cells. Further cytoskeletal correlations of inducible wound-edge activities can be demonstrated by actin staining with phalloidin. The unstimulated cells show a rounded morphology with less polymerized actin bundles (Figure 6C). The EphB1-Fc exposed cells show a stretched-out, bizarre morphology and apparent assembly of stress fibers. The spike-structures may reflect increased focal adhesion assembly (Figure 6D). Furthermore, the enhanced wound closure of the stimulated cells is also accompanied by increased deposition of laminin as a provisional basal membrane at the wound edge (Figure 6E, F).
This study demonstrates a complex spectrum of Eph-RTK and ephrin expression in the normal human adult intestinal mucosa. This suggests that Eph-RTKs and ephrins may contribute to tissue maintenance throughout life, in addition to their welle-stablished roles in development[23]. Consistent with a previous study by Podolsky and co-workers[16], we found EphA2 and ephrin-A1 among the most prominently expressed family members both in the small intestine and colon. To the best of our knowledge, this is the first study showing a concurrent prominent co-expression of EphB2 and ephrin-B1/2 in normal human intestine. Other family members revealed a less abundant expression. This gut ‘signature’ is unique among other normal tissues in humans[15]. In addition, this study reports a differential regulation of ephrin-B2 in the intestinal epithelium of MC patients. In the light of recent insights into the function of ephrin-B2, its upregulation suggests a possible ephrin-B2-dependent modulation of cytoskeletal dynamics and migration competence of epithelial cells in IBD. The multi-modal interaction of ephrin-B2 with downstream effectors of the cytoskeleton such as focal adhesion kinase (FAK) and cdc42 has been recently demonstrated[24].
Therefore, we addressed possible functional effects of an enhanced EphB/ephrin-B-dependent exchange of intercellular signals on epithelial repair processes. The stimulation of rat IEC-6 cells with recombinant ephrin-B2-binding EphB1-Fc receptors effectively enhanced their wound closure competence at doses below 1 nmol/L. Consistent with this ‘repair phenotype’, EphB1-Fc stimulation induced a concerted migratory response of wound-edge cells. This was documented by early archipelagolike movements at the wound-edge associated with increased stress-fiber polymerization and adoption of a stretched-out morphology with multiple wound-oriented lamellipodia. Furthermore, the increased deposition of laminin provides a new basement for integrin-dependent anchorage and survival of the cells[25]. Although the IEC-6 model suggests mechanisms in favor of enhanced wound healing, any conclusions regarding the in vivo situation have to be drawn with caution. As shown by immunohistochemistry of MC lesions, the physiologic expression gradients extending from the basis of the intestinal crypts to the apical epithelium are apparently lost in MC epithelia. This graded expression is tightly regulated by β-catenin and TCF, and has been shown to be responsible for the spatial separation of the proliferative and differentiated epithelial compartment along the crypt-villus axis during development[14]. Since the expression gradient may be the driving principle of organized cell replacement, from the base to the top along the crypt-villus axis, this ectopic expression in MC could also be deleterious. Finally, the upregulation of ephrin-B2 does not necessarily mean enhanced ephrin-B2-dependent signaling in the epithelium, since counteracting mechanisms (e.g. phosphatases such as FAP-1 and PTP-BL) may be up-regulated as well[26].
Extrapolations on in vivo effects of single family members are also hampered by the complexity involved. Members of this largest class of Eph-RTKs in humans can evolve seemingly disparate effects depending on the cellular system and the relative balance of A vs B receptors and ligands. For instance, Lawrenson et al[27] have reported that ephrin-A5, also in our list of upregulated candidate genes in MC epithelium, can induce de-adhesion of EphA3 expressing 293T and melanoma cells. The contrary has been reported for CaCo2 cells[16]. With PC-3 prostate cancer cells transfected with EphA2, stimulation with ephrin-A1 resulted in an inhibition of cell adhesion and spreading on laminin and fibronectin[28]. EphA2 is also linked with the p53 tumor-suppressor protein family and can induce apoptosis[29]. In contrast, ephrin-B/EphB-interaction can promote integrin-mediated cell attachment in embryonic kidney cells[30], endothelial cells[31], platelets[23] and P19 cells[32]. Obviously, the composition and fine-tuned balance of expressed individual members of this large family is a critical point and might contribute to the movement of intestinal cells along the crypt-villus axis[14], or from the wound margin to the center of epithelial defects.
Nevertheless, therapeutically it could be very interesting to strengthen the effects of the protective members and to weaken the effects of the counteracting members in this system, since our study demonstrates that nM doses can provoke significant effects on wound healing in the IEC-6 cell model. With the discovery of defined peptides that activate or specifically block selected molecules of this family[33], a differentiated biological targeting of this delicately balanced system is possible in the near future. Since T-cell homing and interferon-γ production are also dependent on their Eph-RTK/ephrin environment, strategies could evolve that both regain the epithelial homeostasis and attenuate immunologic responses in IBD patients.
We conclude that the EphB/ephrin-B induced pathways should be placed on the list of targets for future molecular therapies trying to intervene with neomorphogenic pathways in IBD epithelia. However, the specifics of such interventions like potential interactions with the immune system, and the function of numerous partly counteracting family members, have to be better understood first.
The skillful technical assistance and support of Mrs. Nadine Wandtke and Lydia Künzel is gratefully acknowledged.
Co-first-authors: Stefanie Meyer
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Wilson AJ, Gibson PR. Epithelial migration in the colon: filling in the gaps. Clin Sci (Lond). 1997;93:97-108. [PubMed] |
| 2. | Podolsky DK. Healing the epithelium: solving the problem from two sides. J Gastroenterol. 1997;32:122-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 128] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Jacinto A, Martinez-Arias A, Martin P. Mechanisms of epithelial fusion and repair. Nat Cell Biol. 2001;3:E117-E123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 283] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 4. | Dignass AU. Mechanisms and modulation of intestinal epithelial repair. Inflamm Bowel Dis. 2001;7:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Drescher U. Eph family functions from an evolutionary perspective. Curr Opin Genet Dev. 2002;12:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Cowan CA, Henkemeyer M. Ephrins in reverse, park and drive. Trends Cell Biol. 2002;12:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Pasquale EB. The Eph family of receptors. Curr Opin Cell Biol. 1997;9:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 168] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Murai KK, Pasquale EB. 'Eph'ective signaling: forward, reverse and crosstalk. J Cell Sci. 2003;116:2823-2832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 284] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 9. | Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M, Pawson T. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383:722-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 420] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 10. | Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998;21:309-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 882] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 11. | Holder N, Klein R. Eph receptors and ephrins: effectors of morphogenesis. Development. 1999;126:2033-2044. [PubMed] |
| 12. | Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999;13:1055-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 562] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 13. | Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM, McDonald DM, Yancopoulos GD. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol. 2001;230:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 278] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 860] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 15. | Hafner C, Schmitz G, Meyer S, Bataille F, Hau P, Langmann T, Dietmaier W, Landthaler M, Vogt T. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin Chem. 2004;50:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 263] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Rosenberg IM, Göke M, Kanai M, Reinecker HC, Podolsky DK. Epithelial cell kinase-B61: an autocrine loop modulating intestinal epithelial migration and barrier function. Am J Physiol. 1997;273:G824-G832. [PubMed] |
| 17. | Langmann T, Moehle C, Mauerer R, Scharl M, Liebisch G, Zahn A, Stremmel W, Schmitz G. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology. 2004;127:26-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 259] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Langmann T, Mauerer R, Zahn A, Moehle C, Probst M, Stremmel W, Schmitz G. Real-time reverse transcription-PCR expression profiling of the complete human ATP-binding cassette transporter superfamily in various tissues. Clin Chem. 2003;49:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 201] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2716] [Cited by in RCA: 2701] [Article Influence: 112.5] [Reference Citation Analysis (0)] |
| 20. | Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116-5121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9125] [Cited by in RCA: 9128] [Article Influence: 380.3] [Reference Citation Analysis (0)] |
| 21. | Sturm A, Sudermann T, Schulte KM, Goebell H, Dignass AU. Modulation of intestinal epithelial wound healing in vitro and in vivo by lysophosphatidic acid. Gastroenterology. 1999;117:368-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Beck PL, Podolsky DK. Growth factors in inflammatory bowel disease. Inflamm Bowel Dis. 1999;5:44-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Prévost N, Woulfe DS, Tognolini M, Tanaka T, Jian W, Fortna RR, Jiang H, Brass LF. Signaling by ephrinB1 and Eph kinases in platelets promotes Rap1 activation, platelet adhesion, and aggregation via effector pathways that do not require phosphorylation of ephrinB1. Blood. 2004;103:1348-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Cowan CA, Henkemeyer M. The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature. 2001;413:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 259] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 25. | Lotz MM, Nusrat A, Madara JL, Ezzell R, Wewer UM, Mercurio AM. Intestinal epithelial restitution. Involvement of specific laminin isoforms and integrin laminin receptors in wound closure of a transformed model epithelium. Am J Pathol. 1997;150:747-760. [PubMed] |
| 26. | Palmer A, Zimmer M, Erdmann KS, Eulenburg V, Porthin A, Heumann R, Deutsch U, Klein R. EphrinB phosphorylation and reverse signaling: regulation by Src kinases and PTP-BL phosphatase. Mol Cell. 2002;9:725-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 229] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Lawrenson ID, Wimmer-Kleikamp SH, Lock P, Schoenwaelder SM, Down M, Boyd AW, Alewood PF, Lackmann M. Ephrin-A5 induces rounding, blebbing and de-adhesion of EphA3-expressing 293T and melanoma cells by CrkII and Rho-mediated signalling. J Cell Sci. 2002;115:1059-1072. [PubMed] |
| 28. | Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol. 2000;2:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 431] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 29. | Dohn M, Jiang J, Chen X. Receptor tyrosine kinase EphA2 is regulated by p53-family proteins and induces apoptosis. Oncogene. 2001;20:6503-6515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Huynh-Do U, Stein E, Lane AA, Liu H, Cerretti DP, Daniel TO. Surface densities of ephrin-B1 determine EphB1-coupled activation of cell attachment through alphavbeta3 and alpha5beta1 integrins. EMBO J. 1999;18:2165-2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 162] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Huynh-Do U, Vindis C, Liu H, Cerretti DP, McGrew JT, Enriquez M, Chen J, Daniel TO. Ephrin-B1 transduces signals to activate integrin-mediated migration, attachment and angiogenesis. J Cell Sci. 2002;115:3073-3081. [PubMed] |
| 32. | Stein E, Lane AA, Cerretti DP, Schoecklmann HO, Schroff AD, Van Etten RL, Daniel TO. Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses. Genes Dev. 1998;12:667-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 334] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 33. | Koolpe M, Dail M, Pasquale EB. An ephrin mimetic peptide that selectively targets the EphA2 receptor. J Biol Chem. 2002;277:46974-46979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 170] [Article Influence: 7.4] [Reference Citation Analysis (0)] |