Published online Jul 14, 2005. doi: 10.3748/wjg.v11.i26.4013
Revised: December 15, 2004
Accepted: December 20, 2004
Published online: July 14, 2005
AIM: Ribavirin (RBV) shows a strong antiviral effect on hepatitis C virus when used in combination with interferon. However, RBV-induced anemia is a major problem in this therapy. It would be of great clinical importance to ameliorate the anemia without reducing the RBV dose. We report here that, Ninjinyoeito (NYT), a herbal medicine can reduce the RBV-induced anemia.
METHODS: Twenty-three patients with chronic hepatitis C were treated with interferon alpha 2b plus RBV with (NYT group) or without (control group) NYT by a randomized selection. Eighteen patients completed the treatment schedule, and hemato-biochemical and virological effects were evaluated.
RESULTS: There was no significant difference in biochemical and virological responses between the two groups. However, anemia was significantly reduced in the NYT group compared with the control group. The maximal decrease of Hb in the NYT group (2.59 ± 1.10 g/dL) was significantly (P = 0.026) smaller than that in the control group (3.71 ± 0.97 g/dL). There was no significant difference in serum glutathione peroxidase activity, serum RBV concentration, and Th1/Th2 balance between the two groups. There was no specific adverse effect in NYT administration.
CONCLUSION: These results suggest that NYT could be used as a supportive remedy to reduce the RBV-induced anemia in the treatment of chronic hepatitis C.
- Citation: Motoo Y, Mouri H, Ohtsubo K, Yamaguchi Y, Watanabe H, Sawabu N. Herbal medicine Ninjinyoeito ameliorates ribavirin-induced anemia in chronic hepatitis C: A randomized controlled trial. World J Gastroenterol 2005; 11(26): 4013-4017
- URL: https://www.wjgnet.com/1007-9327/full/v11/i26/4013.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i26.4013
Chronic hepatitis C often gradually progresses to cirrhosis, and hepatocellular carcinoma develops especially in advanced hepatic fibrosis[1]. Recently, ribavirin (RBV) has been used in combination with pegylated interferon (IFN) as the most effective antiviral therapy[2]. However, RBV-induced anemia is the major dose-limiting factor in the combined therapy of RBV and IFN products[3]. The pathogenesis of RBV-induced anemia is reported to be the destruction of erythrocytes due to the retention of RBV inside the erythrocytes because there is no RBV dephosphorylase in erythrocytes[4], possibly leading to the oxidative damage to the erythrocytic membrane. When anemia is progressive, the dose of RBV is usually reduced, and even the IFN-RBV therapy itself might be stopped in case of severe anemia. If we could prevent or at least reduce RBV-induced anemia to some extent, patients would receive a sufficient amount of RBV and could show a favorable virological response. There have been only a few reports on the attempt to ameliorate the RBV-induced anemia[5,6].
Ninjinyoeito (NYT), a herbal medicinal formula, shows antiviral action on hepatitis C virus (HCV)[7], as well as antioxidant[8], immunomodulatory[9], and hematopoietic[10-12] effects. We speculated that the antioxidant effect of NYT could protect the erythrocytic membrane from the RBV-induced hemolysis and that NYT would promote erythropoiesis in response to the RBV-induced anemia.
In this report, we aimed to elucidate a beneficial effect of NYT on the RBV-induced anemia by conducting a randomized controlled trial.
Patients with chronic hepatitis C were selected for the treatment protocol with the following criteria: serum levels of HCV-RNA more than 100 kIU/mL by RT-PCR (or more than 1 Meq/mL by a branched DNA probe assay) in naive patients, or ineffective or recurrent after the interferon monotherapy. Patients were excluded due to the following criteria: pregnant women, allergic to interferon, administration of a herbal medicine “Shosaikoto”, severe heart failure, chronic renal failure, and past history of depression. Twenty- three patients with chronic hepatitis C were enrolled into this study. Seventeen were male and six were female. The mean age of the patients was 51.1 (range 26-74). The diagnosis was confirmed histologically with liver biopsy in 13 patients. The other patients were diagnosed by hematological, biochemical, and virological findings.
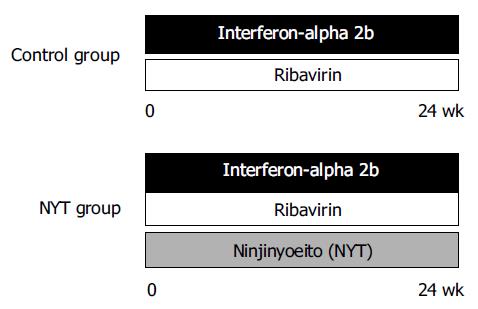
Interferon-alpha 2b (Intron A®, Schering-Plough, Kenilworth, NJ, USA) and RBV (Rebetol®, Schering-Plough) were used as antiviral agents as previously described[6,7]. In principle, interferon-alpha 2b (IFN) was administered intramuscularly 6 d a week for 2 wk at a dose of 10 MU, then thrice a week for 22 wk at the same dose. RBV was administered orally for 24 wk at a dose of 800 mg per day when the patient had a body weight over 60 kg, and at a dose of 600 mg when under 60 kg. Ninjinyoeito extract granules (NYT, Chinese name: Renshen-yangrong-tang, TJ-108, Tsumura & Co., Tokyo, Japan), including 6.0 g of NYT extract, was administered orally for 24 wk at a dose of 9 g per day. The patients were assigned into two groups in a random selection (Figure 1). The NYT group was treated with the three agents (IFN, RBV, and NYT), and the control group was treated with the two agents (IFN and RBV). No specific drugs were administered after the 24-wk treatment. A written informed consent was obtained from all the patients enrolled. Criteria for dose-down or stopping of the IFN and RBV were as follows: criteria by hemoglobin (Hb): dose-down of RBV from 800 to 600 mg or from 600 to 400 mg/d without the change of IFN dose when Hb less than 10 g/dL; administration of both IFN and RBV were stopped when Hb less than 8.5 g/dL; criteria by WBC and platelet counts: dose down of IFN without the change of RBV dose when WBC counts were less than 1 500/µL or platelet counts were 50 000/µL; administration of both IFN and RBV were stopped when WBC counts were less than 1 000/µL or platelet counts were 25 000/µL. Other criteria for dose-down or discontinuation of treatment included subjective symptoms such as anorexia, general malaise, or depressive tendency.
Approximately 20 mL of blood was taken from the patients before treatment and again at 2, 4, 8, 12, and 24 wk after the start of treatment, and 24 wk after the end of treatment. Hematological, biochemical, and virological tests were performed.
Serum HCV-RNA was detected with an Amplicor HCV test (Roche Diagnostics, Tokyo, Japan), and was quantified using an Amplicor HCV Monitor test, version 2.0, Roche Diagnostics). Serotypes of HCV were determined using an enzyme immunoassay (Genotyping EIA, International Reagents, Kobe, Japan). Sustained virological response (SVR) was considered to be obtained when the serum HCV-RNA was negative and serum ALT was within normal limits at 24 wk from the end of the IFN/RBV treatment.
RBV concentration was determined in 18 patients, using high performance liquid chromatography (HPLC, SRL, Tokyo, Japan). Two-hundred microliters of serum was applied to HPLC after deproteination. The range of this assay system was from 50 to 20000 ng/mL.
Serum levels of erythropoietin concentration were determined in eight male patients at wk 0 and 4 (four patients from each group), using radioimmunoassay (SRL, Inc., Tokyo, Japan). The eight patients were selected because their sera were available and it was considered to be adequate to compare the male patients only instead of mixing a few female patients.
The Th1/Th2 balance as a marker for cellular immunological response was assayed using the flow cytometry-based “Fastimmune Cytokine System” (Beckton Dickinson Biosciences, San Jose, CA, USA). Briefly, the blood was collected in sodium heparin, and was activated with ionomycin (IM), Brefeldin-A (BFA), and phorbol 12-myristate 13 acetate (PMA). After lysing and permeabilization, intracellular staining with fluorescent-conjugated monoclonal antibodies against cytokines was done. The two-color interferon-γ/interleukin-4 staining (Fastimmune system, Beckton Dickinson Biosciences) was utilized. Laser flow cytometer (FACS Caliber, Beckton Dickinson Biosciences) was used for analysis.
Glutathione peroxidase activity as an antioxidant activity was determined using the Glutathione Peroxidase Assay kit (Cayman Chemical Company, Ann Arbor, MI, USA).
The software package Statmate III (Atms, Tokyo, Japan) was used for statistical analysis. The two-tailed Mann-Whiney U test was used to assess the significance of difference between the various laboratory test items such as Hb. The Fisher’s exact test was used for the comparison of antiviral effects between the two groups. Multiple regression analysis was performed for the analysis on the factors influencing the anemia. All data were expressed as the mean±SD. A P value less than 0.05 was considered to be statistically significant.
Patients were treated with IFN plus RBV either with (n = 13) or without (n = 10) NYT. Eighteen patients (nine patients in each group) completed the 24-wk treatment. There were five patients who dropped out due to anorexia and general malaise in three cases (all in the control group) and due to social problems in two cases (one case in each group). There was no significant difference between the NYT and control groups in age, gender, body weight, serum ALT levels, Hb levels, HCV-RNA levels before treatment, and HCV serotypes (Table 1). During the course of treatment, the dose of RBV was stopped in 1 patient, but not reduced in any of the remaining 9 patients in the NYT group, whereas it was reduced due to anemia in 2 of 13 patients, resulting in stopping of RBV in 4 patients in the control group (P = 0.45).
| NYT | Control | P | |
| Age (yr) | 46.3 ± 14.3 | 54.9 ± 10.3 | 0.113 |
| Gender (M/F) | 9/1 | 8/5 | 0.123 |
| Body weight (kg) | 65.2 ± 9.8 | 60.6 ± 9.1 | 0.291 |
| ALT (U/L) | 74.9 ± 38.2 | 58.1 ± 25.1 | 0.457 |
| Hb ( g/dL) | 14.3 ± 1.4 | 14.0 ± 1.6 | 0.709 |
| HCVRNA (kIU/mL) | 553.5 ± 315.0 | 637.6 ± 314.0 | 0.673 |
| HCV serotype (1/2) | 8/2 | 9/4 | 0.377 |
There was no significant difference in RBV concentrations at wk 4 between the two groups. In the intention-to-treat analysis as in Table 2, serum ALT levels were within normal limits in 7 of 10 (70.0%) patients in the NYT group whereas they were normal in 6 of 13 (46.2%) patients in the control group at the end of the IFN/RBV treatment (P = 0.253). At the same time point, HCV-RNA turned negative in 8 of 10 (80.0%) patients in the NYT group whereas it was negative in 7 of 13 (53.8%) patients in the control group (P = 0.192). At 6 mo after the NYT/RBV treatment, serum ALT levels were within normal limits in 8 of 10 (80.0%) patients in the NYT group while they were within normal limits in 4 of 13 (30.8%) patients in the control group (P = 0.489). Sustained virological response (SVR) was obtained in 4 of 10 (40.0%) patients in the NYT group whereas it was seen in 4 of 13 (30.8%) in the control group (P = 0.645). In patients with serotype 1, SVR was obtained in 3 of 8 (37.5%) patients in the NYT group and 1 of 9 (11.1%) patients in the control group (P = 0.311), whereas in patients with serotype 2, SVR was recognized in 2 of 2 (100%) in the NYT group and 3 of 4 (75%) in the control group (P = 0.316).
| NYT (%) | Control (%) | P | |
| Enrolled cases | 10 | 13 | |
| Normal ALT at the end of therapy | 7 (70.0) | 6 (46.2) | 0.253 |
| Negative HCVRNA at the end of therapy | 8 (80.0) | 7 (53.8) | 0.192 |
| Normal ALT 6 mo after therapy | 8 (80.0) | 4 (30.8) | 0.489 |
| Negative HCVRNA 6 mo after therapy | 4 (40.0) | 4 (30.8) | 0.645 |
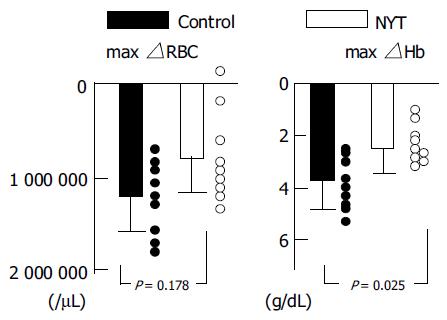
We analyzed the hematological changes in the 18 patients who received the IFN/RBV treatment for more than 4 wk. As shown in Table 3 and Figure 2, the maximal decrease of Hb in the NYT group (2.59 ± 1.10 g/dL) was significantly (P = 0.026) smaller than that in the control group (3.71 ± 0.97 g/dL). The minimal Hb during the treatment in the NYT group (11.66 ± 1.78 g/dL) tended to be higher than that in the control group (10.10 ± 1.08 g/dL, P = 0.079). Maximal decrease of erythrocytes in the NYT group (868 000 ± 474 000/μL) tended to be smaller than that (1244000 ± 419000/μL) in the control group (P = 0.178). As shown in Table 1, there were some differences, although not significant, in age, gender, and body weight, which might influence the grade of anemia. We performed a multivariate analysis using the multiple regression method. Backward stepwise regression was performed, and a P value greater than 0.20 was used for a variable removal, and the NYT administration and serum creatinine were picked up. The final linear regression model selected only the NYT administration as a significant variable (P = 0.0042) influencing maximal hemoglobin decrease (Table 4). There were no significant differences in the WBC or platelet (Plts) counts between the two groups.
| NYT | Control | P | |
| Max△RBC (× 103) | 868 ± 474 | 1.244 ± 419 | 0.178 |
| Max△Hb (g/dL) | 2.59 ± 1.10 | 3.71 ± 0.97 | 0.026 |
| min Hb (g/dL) | 11.66 ± 1.78 | 10.10 ± 1.08 | 0.079 |
| WBC (wk 24) | 3 444 ± 1731 | 3 204 ± 1143 | 0.894 |
| Plts (wk 24, × 104) | 14.69 ± 5.52 | 12.77 ± 4.22 | 0.709 |
| Th1 (wk 4, %) | 27.29 ± 11.6 | 30.20 ± 8.66 | 0.480 |
| Th2 (wk 4, %) | 2.77 ± 1.06 | 2.30 ± 1.09 | 0.232 |
| Th1/Th2 (wk 4) | 10.89 ± 5.68 | 15.48 ± 6.87 | 0.130 |
| GP×[wk 4, nmol/(min·mL)] | 76.41 ± 31.79 | 78.96 ± 31.96 | 0.522 |
| Variable | Parameter estimate | Standard error | P |
| Intercept | -1.61742 | 1.12391 | 0.1707 |
| NYT administration | 1.47645 | 0.43746 | 0.0042 |
| Serum creatinine (wk 0) | -3.05400 | 1.57891 | 0.0722 |
Serum levels of erythropoietin were determined in eight male patients at wk 0 and 4 (four patients from each group), and there was no significant difference in the increase of erythropoietin from wk 0 to wk 4 between the two groups (38.4 ± 21.6 ng/mL in the NYT group vs 45.2 ± 30.3 ng/mL in the control group, P = 0.885).
There were no significant changes in Th1, Th2, or the Th1/ Th2 ratio in either group during the observation period in each group, and there were no significant differences in these cellular immunological markers between the two groups at any time point. Table 3 shows the results at wk 4.
There were no significant changes in glutathione peroxidase activity during the observation period, and there was no significant difference in this activity between the two groups at any time point. Table 3 shows the results at wk 4.
In this study, we elucidated, for the first time, the anemia-ameliorating effect of NYT in the IFN/RBV treatment for chronic hepatitis C in a randomized controlled trial. Although NYT possesses a hematopoietic action[10-12], we speculate that anemia-reducing effect of NYT was derived from its anti-oxidant[8] and stabilizing actions on the erythrocytic membrane, ameliorating the hemolytic anemia. NYT is reported to show an anti-HCV action[7], but NYT did not affect the anti-viral effect of IFN/RBV therapy in this study. If we can reduce the grade of anemia and discontinuation of therapy, it would lead to the increase in number of patients with SVR.
As mentioned above, NYT is reported to have the following effects: hematopoietic[10-12], antioxidant[8], hepatocyte-protective[13], Th1/Th2 balancing[9], and direct anti-HCV[7]. NYT has been used in the treatment of asthenic or fatigued patients, especially after operation or severe infectious diseases[14]. NYT is effective for various signs and symptoms, including anemia, general malaise, anorexia, night sweating, sensation of cold in the extremities, cough, and insomnia[14]. These effects have been analyzed and verified in the theory of Traditional Chinese Medicine. From the viewpoint of hepatitis treatment, these effects of NYT are suitable for ameliorating the adverse effects of IFN (general malaise, anorexia, and psychiatric problems) and that of RBV (anemia). The dose of NYT was 9.0 g/d in this study, which is a normal dose in Japan.
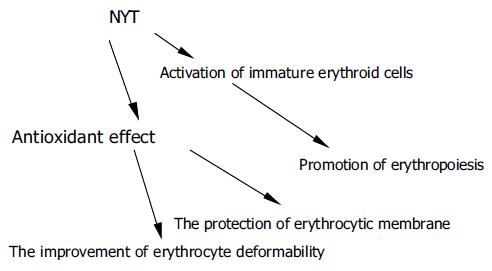
The precise mechanisms of RBV-induced anemia are unknown, but several reports[3,4] suggest that the accumulation of phosphorylated RBV in erythrocytes due to the lack of dephosphorylase in erythrocytes impairs the deformability of erythrocytes. The accumulated RBV may affect the antioxidant defense mechanisms of the erythrocytic membrane. These changes in erythrocytes would lead to the extravascular destruction of erythrocytes (hemolysis). Since NYT has an antioxidant action, it may show a beneficial effect on the erythrocytic membrane. Although NYT would not affect the accumulated RBV inside the erythrocyte, NYT might improve erythrocytic deformability in a different manner, like other herbal medicines with common components. Therefore, the reasons why NYT ameliorates RBV-induced anemia might include the protection of erythrocytic membrane, the improvement of erythrocytic deformability, and erythropoiesis (Figure 3).
Previous reports showed that erythropoietin[5] and eicosapentaenoic acid[6] can be effective against RBV-induced anemia. In the prospective, double-blind, randomized controlled study by Afdhal et al[5] in 185 patients, erythropoietin (EPO) was administered after anemia developed, and the mean Hb increase was 2.2 ± 1.3 g/dL, which was significantly greater than 0.1 ± 1.0 g/dL in the placebo group. However, the use of EPO would be expensive and would not address the solution of hemolysis. In the uncontrolled, pilot study by Ide et al[6] in six patients, eicosapentaenoic acid (EPA) was also administered after the development of anemia, and the mean Hb significantly increased from 10.8 to 11.4 g/dL in 1 mo. The effect of EPA seems to be marginal, and it was tested in a pilot study and would need a randomized trial. EPA improves erythrocyte deformability and increases EPA content in erythrocytic membrane phospholipids[6]. Table 5 summarizes the comparison among NYT, EPO, and EPA from the viewpoints of effectiveness, adverse reactions, and cost-benefits. EPO is expensive, with more adverse effects, but most effective. NYT is economical, with little adverse reaction. EPA seems to be intermediate between the above two drugs.
| Action | Mechanisms | Indication for anemia in health | Form | Adverse reactions | Daily cost | |
| insurance system of Japan | (yen) | |||||
| NYT | Promotion of erythropoiesis, | Activation of immature | Anemia in | Granule | Abdominal discomfort | 227.7 |
| protection of erythrocytic | erythroid cells, | general, | (frequency unknown) | |||
| membrane, improvement of | antioxidant effect | |||||
| erythrocytic deformability | ||||||
| EPA | Increase in erythrocyte | Increase in EPA content in | None | Capsule | Jaundice, gynecomastia | 353.4 |
| deformability | erythrocytic membrane | (frequency unknown), | ||||
| phospholipids | hemorrhagic tendency, | |||||
| liver injury (< 0.1%) | ||||||
| EPO | Increase in erythropoiesis | Stimulation of stem cell | Renal anemia | Vial for | Thromboembolism, | 1643 |
| differentiation to | injection | cerebral hemorrhage, | (750 IU) | |||
| erythroid cells | shock (frequency unknown), | |||||
| high blood pressure, liver | ||||||
| injury (0.1-< 5%) |
In our study, NYT was administered from the beginning of IFN/RBV treatment, and we cannot simply compare the results between the above-mentioned studies and ours. We would like to test whether NYT improves the RBV-induced anemia after it occurs, or whether pretreatment with NYT prevents the anemia.
Erythropoietin response is reported to be subnormal in the IFN/RBV treatment. Although NYT has a bone marrow-stimulatory action via enhancing colony stimulating factors[11], there was no significant difference in the endogenous erythropoietin response between the NYT and control groups in our study. Therefore, NYT might ameliorate RBV-induced anemia, not by stimulating hematopoiesis, but by reducing hemolysis. We measured serum glutathione peroxidase activity before and after the IFN/RBV therapy with or without NYT. There was no significant change both in time course and between the NYT and control groups. But, according to the previous reports[8,13] it is likely that NYT may exert its protective effect on the erythrocytic membrane by antioxidative action and membrane stabilization. Serum antioxidant markers other than glutathione peroxidase might reveal the effect of NYT.
Regarding the combination therapy of IFN and herbal medicines, Shosaikoto (SST, Shao-chaihu-tang) is prohibited for use in combination with IFN because this combination is reported to increase the risk of interstitial pneumonia. The herbal component Scutellariae radix alone does not cause interstitial pneumonia, but when used in combination with other herbal components, some unknown factors causing interstitial pneumonia are considered to be expressed. NYT does not contain five of the seven medicinal herbs used in SST, including Scutellariae radix. Interstitial pneumonia or any other adverse reactions in relation to NYT use were not found in this study. Since NYT was used safely in the IFN/RBV therapy, NYT could be used in combination with PEG-IFN+RBV for 48 wk, which is considered as the most effective anti-HCV therapy[2]. This current worldwide standard was not available during the period of this study in Japan.
In conclusion, NYT ameliorated the RBV-induced anemia in RCT. NYT did not show any stronger antiviral effect on HCV, but it was used safely without any adverse reaction in the combination treatment with IFN/RBV. These results provide good evidence for the effect of the herbal medicine NYT. It is suggested that NYT can be used as a supportive drug for IFN/RBV therapy for chronic hepatitis C. Active mechanisms are to be elucidated and these results would be verified in additional studies with more patients.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Gish RG. Treating hepatitis C: the state of the art. Gastroenterol Clin North Am. 2004;33:S1-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Hadziyannis SJ, Sette H, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H, Bernstein D, Rizzetto M. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2216] [Cited by in RCA: 2109] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 3. | De Franceschi L, Fattovich G, Turrini F, Ayi K, Brugnara C, Manzato F, Noventa F, Stanzial AM, Solero P, Corrocher R. Hemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: role of membrane oxidative damage. Hepatology. 2000;31:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 328] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 4. | Page T, Connor JD. The metabolism of ribavirin in erythrocytes and nucleated cells. Int J Biochem. 1990;22:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Afdhal NH, Dieterich DT, Pockros PJ, Schiff ER, Shiffman ML, Sulkowski MS, Wright T, Younossi Z, Goon BL, Tang KL. Epoetin alfa maintains ribavirin dose in HCV-infected patients: a prospective, double-blind, randomized controlled study. Gastroenterology. 2004;126:1302-1311. [PubMed] |
| 6. | Ide T, Okamura T, Kumashiro R, Koga Y, Hino T, Hisamochi A, Ogata K, Tanaka K, Kuwahara R, Seki R. A pilot study of eicosapentaenoic acid therapy for ribavirin-related anemia in patients with chronic hepatitis C. Int J Mol Med. 2003;11:729-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 7. | Cyong JC, Ki SM, Iijima K, Kobayashi T, Furuya M. Clinical and pharmacological studies on liver diseases treated with Kampo herbal medicine. Am J Chin Med. 2000;28:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Egashira T, Takayama F, Komatsu Y. Changes of materials that scavenge 1,1-diphenyl-2-picrylhydrazyl radicals in plasma by per-oral administration of Kampo medicine, Ninjin-yoei-to in rats. J Pharm Pharmacol. 2003;55:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Nakada T, Watanabe K, Jin GB, Triizuk K, Hanawa T. Effect of ninjin-youei-to on Th1/Th2 type cytokine production in different mouse strains. Am J Chin Med. 2002;30:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Miura S, Kawamura I, Yamada A, Kawakita T, Kumazawa Y, Himeno K, Nomoto K. Effect of a traditional Chinese herbal medicine ren-shen-yang-rong-tang (Japanese name: ninjin-youei-to) on hematopoietic stem cells in mice. Int J Immunopharmacol. 1989;11:771-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Okamura S, Shimoda K, Yu LX, Omori F, Niho Y. A traditional Chinese herbal medicine, ren-shen-yang-rong-tang (Japanese name: ninjin-yoei-to) augments the production of granulocyte-macrophage colony-stimulating factor from human peripheral blood mononuclear cells in vitro. Int J Immunopharmacol. 1991;13:595-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Fujii Y, Imamura M, Han M, Hashino S, Zhu X, Kobayashi H, Imai K, Kasai M, Sakurada K, Miyazaki T. Recipient-mediated effect of a traditional Chinese herbal medicine, ren-shen-yang-rong-tang (Japanese name: ninjin-youei-to), on hematopoietic recovery following lethal irradiation and syngeneic bone marrow transplantation. Int J Immunopharmacol. 1994;16:615-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Takayama F, Egashira T, Yamanaka Y. Protective effect of Ninjin-yoei-to on damage to isolated hepatocytes following transient exposure to tert-butyl hydroperoxide. Jpn J Pharmacol. 2001;85:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Terasawa K. Ninjin-yoei-to. Kampo: Japa-nese-Oriental Medicine: Insights from clinical cases. 1st ed. Tokyo: K.K. Standard McIntyre 1993; 242. |