Published online Jul 14, 2005. doi: 10.3748/wjg.v11.i26.4008
Revised: June 20, 2004
Accepted: June 24, 2004
Published online: July 14, 2005
AIM: To study the effect of caffeic acid phenethyl ester (CAPE) on proliferation, cell cycle, apoptosis and expression of β-catenin in cultured human colorectal cancer (CRC) cell line HCT116.
METHODS: HCT116 cells were treated with CAPE at serial concentrations of 80, 40, 20, 10, 5, 2.5 mg/L. The proliferative status of HCT116 cells was measured by using methaben-zthiazuron (MTT) assay. Cell cycle was analyzed by using flow cytometry (FCM) with propidium iodide (PI) labeling method. The rate of apoptosis was detected by using FCM with annexin V-FITC and PI double labeling method. β-catenin levels were determined by Western blotting. β-catenin localization in HCT116 was determined by indirect immunofluorescence.
RESULTS: After HCT116 cells were exposed to CAPE (80, 40, 20, 10, 5, and 2.5 mg/L) for 24, 48, 72, 96 h, CAPE displayed a strong growth inhibitory effect in a dose- and time-dependent manner against HCT116 cells. FCM analysis showed that the ratio of G0/G1 phase cells increased, S phase ratio decreased and apoptosis rate increased after HCT116 cells were exposed to CAPE (10, 5, and 2.5 mg/L) for 24 h. CAPE treatment was associated with decreased cytoplasmic β-catenin, nuclear β-catenin and a concurrent increase in β-catenin protein expression at cell-cell junctions.
CONCLUSION: CAPE could inhibit HCT116 cell proliferation and induce cell cycle arrest and apoptosis. Decreased β-catenin protein expression may mediate the anti-proliferative effects of CAPE.
- Citation: Wang D, Xiang DB, He YJ, Li ZP, Wu XH, Mou JH, Xiao HL, Zhang QH. Effect of caffeic acid phenethyl ester on proliferation and apoptosis of colorectal cancer cells in vitro. World J Gastroenterol 2005; 11(26): 4008-4012
- URL: https://www.wjgnet.com/1007-9327/full/v11/i26/4008.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i26.4008
Colorectal cancer (CRC) is one of the most common human malignancies, and is the fourth leading cause of cancer deaths. The incidence tends to increase in China. Aberrant WNT pathway signaling is an early progression event in 90% of colorectal cancers. It occurs through mutations mainly of APC and less often of CTNNB1 (encoding β-catenin) or AXIN2 (encoding axin-2, also known as conductin). Activation of WNT signaling leads to inhibition of GSK-3β activity, resulting in accumulation of cytoplasmic (signaling) β-catenin, which becomes available to bind the TCF/LEF family of transcription factors and to induce target gene expression[1]. Dysregulation of WNT signaling and hence β-catenin expression is believed to be central to the early stages of sporadic colorectal carcinogenesis in human beings[2]. Therefore, control of β-catenin and/or control of downstream TCF target gene expression represents an ideal target for CRC chemoprevention. Caffeic acid phenethyl ester (CAPE), an active component of propolis, has many biological and pharmacological activities including antioxidant, anti-inflammation, antiviral action, anticancer effect, etc.[3-7]. It also inhibited the development of azoxymethane-induced aberrant crypts in the colon of rats[8]. Thus, we investigated the effects of CAPE on proliferation, cell cycle, apoptosis and expression of β-catenin in the cultured human colorectal cancer (CRC) cell line HCT116.
CAPE, dimethyl sulphoxide (DMSO), PI and MTT were purchased from Sigma-Aldrich (USA). RPMI-1640 medium was purchased from Hyclone. Mouse monoclonal anti-human β-catenin antibody was obtained from Sigma-Aldrich (USA). FITC and horseradish peroxidase-conjugated secondary antibody were obtained from Pierce Biotechnology (USA). Annexin-V and PI double staining kit was purchased from Roche (USA). CAPE was prepared as 80 g/L stock solutions in DMSO. Control flasks or plates contained DMSO at an equivalent dilution to that in cultures containing CAPE.
The human CRC cell line HCT116 was purchased from the American Type Culture Collection (ATCC). The cells were cultured in RPMI1640 medium (pH 7.2-7.4) supplemented with penicillin G (100 U/mL), streptomycin (100 U/mL) and 10% fetal calf serum (FCS) at 37°C in a 50 mL/L CO2 humidified atmosphere. Cells were routinely sub-cultured using 2.5 g/L trypsin/ethylenedinitrile tetraacetic acid (EDTA) solution (obtained from Sigma).
The logarithmically growing HCT116 cells were plated at a density of 4×103 cells/well into a 96-well plate. After 24 h, cells were treated with CAPE at various concentrations (80, 40, 20, 10, 5, and 2.5 mg/L) for 24, 48, 72, and 96 h. Control wells were treated with 0.1% DMSO alone. Then, 20 μL MTT (5 g/L) was added to each well and incubated for an additional 4 h, and then culture media were discarded followed by addition of 0.15 mL DMSO and vibrated for 10 min. The absorbance was measured at 490 nm using a model 550 microplate reader. The inhibitory rates (IR) were calculated as follows: IR (%) = (1-absorbance of the treated wells)/(absorbance of the control wells) ×100%. The 50% inhibitory concentration (IC50) value was determined using a CalcuSyn software.
The cells were grown at 50–60% confluence in T75 flasks, serum-starved for 24 h and then treated with a range of CAPE (10, 5, and 2.5 mg/L) for 24 h. At the end of the treatment, the floating cells were collected by centrifugation, whereas adherent cells were harvested by trypsin-EDTA solution to produce a single cell suspension. The cells were then pelleted by centrifugation and washed twice with PBS. Then, the cell pellets were suspended in 0.5 mL PBS and fixed in 5 mL ice-cold 70% ethanol at 4°C. The fixed cells were spun by centrifugation and the pellets were washed with PBS. After resuspension with 1 mL PBS, the cells were incubated with RNase A (20 mg/L) and PI (50 mg/L) and shaken for 1 h at 37°C in the dark. The stained cells were analyzed using a FACScan flow cytometer in combination with BD lysis II software (Becton Dickinson).
The cells were treated and harvested as above. Annexin-V and PI double staining kit (Roche) was used to assess apoptosis, then cells were immediately analyzed by flow cytometry. Early apoptotic cells were localized in the lower right quadrant of a dot-plot graph using annexin-V-fluorescein vs PI.
Cells grown on glass coverslips were treated for 24 h with CAPE (10, 5, and 2.5 mg/L) or an equivalent dilution of DMSO, under standard culture conditions as described above. Cells were fixed immediately in 4% formaldehydum polymerisatum at 20°C for 60 min and washed twice in PBS. Apoptosis of HCT-116 cells was evaluated by using an in situ cell death detection kit (Roche Co. Ltd, Germany). Monolayers were treated with proteinase K and then 0.3% H2O2, labeled with fluorescein dUTP in a humidified box for 1 h at 37°C. The cells were then combined with POD-horseradish peroxidase, colorized with diaminobenzidine tetrahydrochloride (DAB). Controls consisted of omission of fluorescein dUTP. Cells were visualized under a light microscope.
Cells were seeded into flasks and grown to 50-60% confluence for 24 h. The cells were then placed in serum-free medium with or without CAPE (10, 5, and 2.5 mg/L) for a period of 24 and 48 h. In the end, the attached cells and floating cells were extracted in lysis buffer using standard methods. Western blot was carried out using standard techniques. Briefly, equal amounts of proteins in each sample were resolved in 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and the proteins transferred onto PVDF membranes. After blocking with non-fat dried milk, the membranes were incubated with the appropriate dilution of primary antibody (mouse monoclonal anti-human β-catenin antibody). The membranes were then incubated with a horseradish peroxidase-conjugated secondary antibody. The proteins were detected by an enhanced chemiluminescence detection system, and light emission was captured on Kodak X-ray films.
Cells grown on glass coverslips were treated for 24 and 48 h with CAPE (10, 5, and 2.5 mg/L) or an equivalent dilution of DMSO, under standard culture conditions as described above. Cells were fixed in 100% methanol at -20°C for 10 min and washed twice in PBS. Monolayers were incubated with primary antibody (mouse monoclonal anti-human β-catenin antibody) in PBS plus 10 g/L dried skimmed milk powder overnight at 4°C. Omission of the respective primary antibody was used as a negative control. Monolayers were incubated with FITC-conjugated secondary antibody in 1% dried skimmed milk in PBS for 30 min at 37°C. Confocal microscopy was performed using a Leica TCS SP laser scanning confocal microscope.
Data were expressed as mean±SD. Analysis of data was performed using one-way ANOVA. P < 0.05 was considered statistically significant.
Effect of CAPE on cell proliferation
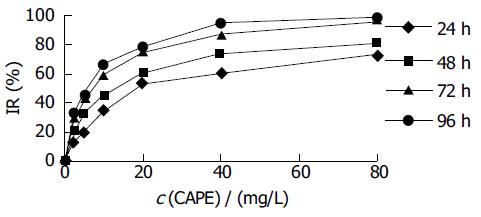
HCT116 cells were treated with CAPE at various concentrations for 1-4 d, and the cell viability was determined as described above by MTT assay. As shown in Figure 1, CAPE inhibited the growth of HCT116 cells in a dose- and time-dependent manner. The IC50 value for CAPE at 24, 48, 72, 96 h after treatment was 22.45, 12.07, 6.47, 5.36 mg/L, respectively.
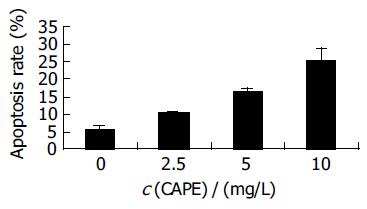
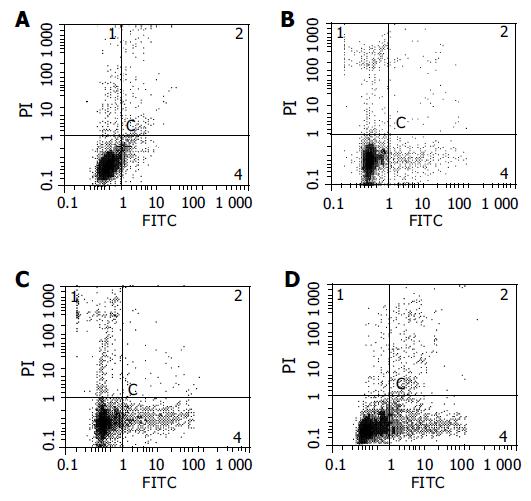
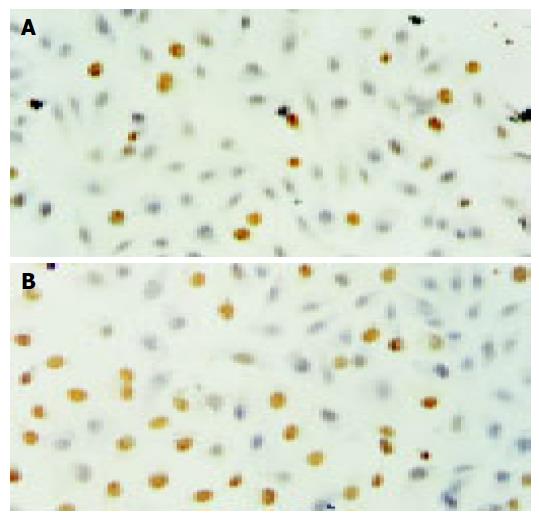
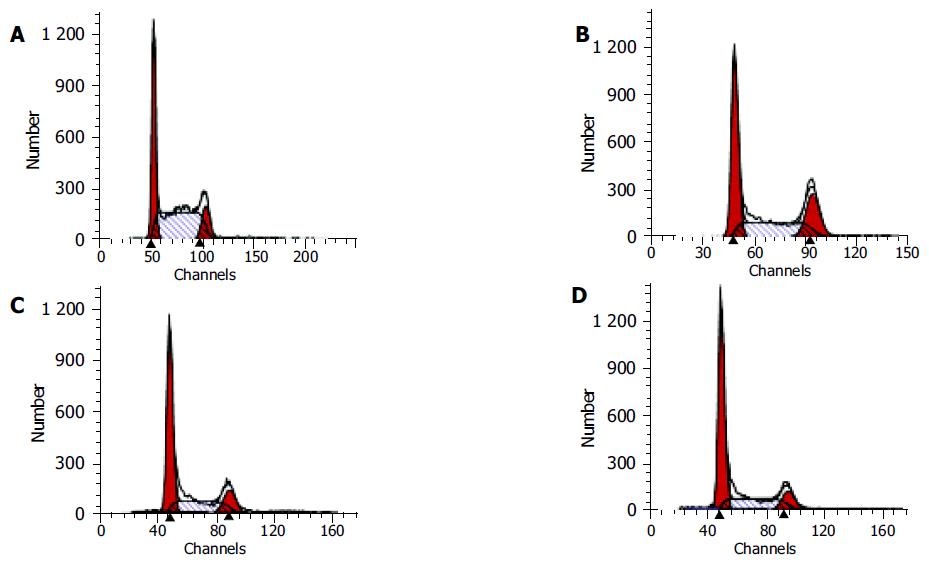
After HCT116 cells were exposed to CAPE (10, 5, and 2.5 mg/L) for 24 h, annexin-V and PI double-staining FCM analysis showed that the apoptosis rates were 25.5% ± 3.3%, 16.6%±0.6%, 10.2% ± 0.7%, respectively, which were significantly higher than control (5.5% ± 0.9%) (P < 0.01). CAPE induced apoptosis in a concentration-dependent manner in HCT116 cells (Figures 2 and 3). TUNEL assay showed that the apoptosis cells were significantly increased in a concentration-dependent manner in HCT116 cells (Figure 4).
After HCT116 cells were exposed to CAPE(10, 5, and 2.5 mg/L) for 24 h, FCM analysis showed that the proportion of cells in G0/G1 phase increased , the proportion of cells in S phase decreased in a concentration-dependent manner (Table 1, Figure 5).
After HCT116 cells were exposed to CAPE (10, 5, and 2.5 mg/L) for 24 and 48 h, Western blot analysis showed that CAPE treatment was associated with a concentration-dependent decrease in β-catenin protein expression in HCT116 cells (Figure 6).
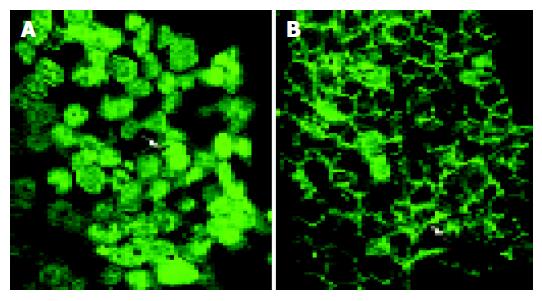
Indirect immunofluorescence studies of β-catenin localization in HCT116 cells revealed that CAPE treatment was associated with decreased cytoplasmic β-catenin, nuclear β-catenin and a concurrent increase in β-catenin protein expression at cell-cell junctions (Figure 7) compared with cells treated with DMSO alone.
Polyphenolic compounds derived from natural products are well known to have various beneficial effects namely anti-tumor[9,10], anti-inflammatory and antioxidant properties[11,12]. CAPE is also a phenolic compound and an active component of honeybee propolis[13,14]. Several investigators have demonstrated that CAPE has anti-proliferative effect, apoptosis-inducing effect against various tumor cell lines[3-7,15] and an antioxidant effect by lipoxygenase inhibition[16]. Also, dietary intake of CAPE decreased tumor formation and expression of the oncoprotein catenin in the enterocytes of the Min/+ mouse[17]. In the present study, we investigated the effect of CAPE on the proliferation, cell cycle and apoptosis of CRC HCT116 cells. Our data demonstrated that CAPE treatment was associated with a strong inhibition of proliferation in a dose- and time-dependent manner, along with induction of G0/G1 arrest and apoptosis in HCT116 cells. Similar to our findings, CAPE entered HL-60 cells very quickly and then inhibited their survival in a concentration- and time-dependent manner. CAPE induced characteristic DNA fragmentation and morphological changes typical of apoptosis in these cells[4].
To investigate the mechanism of the anti-proliferative effects of CAPE on HCT116 cells, we studied the effect of CAPE on the expression of β-catenin, a multifunctional protein, responsible for the transduction of WNT mediated signals as well as for cell-cell adhesion[18,19]. The signaling function of β-catenin is particularly important in colon cancer in which activation of β-catenin, as a result of mutations in APC or stabilizing mutations in the N-terminal region of β-catenin, is common[20]. Activating mutations of β-catenin are also common in many other cancers[21]. β-catenin signaling promotes the G1 to S-phase transition, inhibits anoikis and allows cells to progress into S-phase after radiation damage[22]. WNT-1 signaling also inhibits apoptosis by activation of β-catenin/TCF-mediated transcription[23] and β-catenin signaling plays an important role in the growth of colon cancer cells[24].
In our study, Western blot analysis showed that CAPE treatment was associated with a concentration-dependent decrease in β-catenin protein expression in HCT116 cells. The immunofluorescence data suggest that downregulation of β-catenin protein measured by Western blot analysis following CAPE treatment was associated with decreased cytoplasmic β-catenin, nuclear β-catenin and a concurrent increase in β-catenin protein expression at cell-cell junctions. Multiple molecular mechanisms seem to be involved in the tumor suppressive effects of CAPE. Recently, it is reported that CAPE induced apoptosis via Fas signal activation in human breast cancer MCF-7 cells[25]. Moreover, tumor suppressor protein p53 and p38 MAPK play a prominent role in the CAPE-induced apoptotic cell death which might contribute to the antitumor effects of CAPE in C6 glioma cells[26].
In summary, we have identified that CAPE could inhibit HCT116 cell proliferation and induce cell cycle arrest and apoptosis. Decreased β-catenin protein expression may mediate the anti-proliferative effects of CAPE. The present findings may help to explain some features of CAPE-mediated chemoprevention. Future experiments will tell which β-catenin-related downstream genes may be affected by CAPE and whether CAPE may play a role in chemoprevention strategies.
Science Editor Zhu LH Language Editor Elsevier HK
| 1. | Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286-3305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 1971] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 2. | Behrens J. Control of beta-catenin signaling in tumor development. Ann N Y Acad Sci. 2000;910:21-33; discussion 33-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 180] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Chen YJ, Shiao MS, Wang SY. The antioxidant caffeic acid phenethyl ester induces apoptosis associated with selective scavenging of hydrogen peroxide in human leukemic HL-60 cells. Anticancer Drugs. 2001;12:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 141] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Chen YJ, Shiao MS, Hsu ML, Tsai TH, Wang SY. Effect of caffeic acid phenethyl ester, an antioxidant from propolis, on inducing apoptosis in human leukemic HL-60 cells. J Agric Food Chem. 2001;49:5615-5619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Lee YJ, Liao PH, Chen WK, Yang CY. Preferential cytotoxicity of caffeic acid phenethyl ester analogues on oral cancer cells. Cancer Lett. 2000;153:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Usia T, Banskota AH, Tezuka Y, Midorikawa K, Matsushige K, Kadota S. Constituents of Chinese propolis and their antiproliferative activities. J Nat Prod. 2002;65:673-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Nomura M, Kaji A, Ma W, Miyamoto K, Dong Z. Suppression of cell transformation and induction of apoptosis by caffeic acid phenethyl ester. Mol Carcinog. 2001;31:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Borrelli F, Izzo AA, Di Carlo G, Maffia P, Russo A, Maiello FM, Capasso F, Mascolo N. Effect of a propolis extract and caffeic acid phenethyl ester on formation of aberrant crypt foci and tumors in the rat colon. Fitoterapia. 2002;73 Suppl 1:S38-S43. [PubMed] |
| 9. | Suganuma M, Okabe S, Sueoka N, Sueoka E, Matsuyama S, Imai K, Nakachi K, Fujiki H. Green tea and cancer chemoprevention. Mutat Res. 1999;428:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 139] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Kuroda Y, Hara Y. Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat Res. 1999;436:69-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 316] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 11. | Martinez J, Moreno JJ. Effect of resveratrol, a natural polyphenolic compound, on reactive oxygen species and prostaglandin production. Biochem Pharmacol. 2000;59:865-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 271] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Chan MM, Mattiacci JA, Hwang HS, Shah A, Fong D. Synergy between ethanol and grape polyphenols, quercetin, and resveratrol, in the inhibition of the inducible nitric oxide synthase pathway. Biochem Pharmacol. 2000;60:1539-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Banskota AH, Tezuka Y, Kadota S. Recent progress in pharmacological research of propolis. Phytother Res. 2001;15:561-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 474] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 14. | Murad JM, Calvi SA, Soares AM, Bankova V, Sforcin JM. Effects of propolis from Brazil and Bulgaria on fungicidal activity of macrophages against Paracoccidioides brasiliensis. J Ethnopharmacol. 2002;79:331-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Nagaoka T, Banskota AH, Tezuka Y, Saiki I, Kadota S. Selective antiproliferative activity of caffeic acid phenethyl ester analogues on highly liver-metastatic murine colon 26-L5 carcinoma cell line. Bioorg Med Chem. 2002;10:3351-3359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Ozyurt H, Irmak MK, Akyol O, Söğüt S. Caffeic acid phenethyl ester changes the indices of oxidative stress in serum of rats with renal ischaemia-reperfusion injury. Cell Biochem Funct. 2001;19:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Mahmoud NN, Carothers AM, Grunberger D, Bilinski RT, Churchill MR, Martucci C, Newmark HL, Bertagnolli MM. Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Carcinogenesis. 2000;21:921-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 218] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 792] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 19. | Gottardi CJ, Gumbiner BM. Adhesion signaling: how beta-catenin interacts with its partners. Curr Biol. 2001;11:R792-R794. [PubMed] |
| 20. | Bright-Thomas RM, Hargest R. APC, beta-Catenin and hTCF-4; an unholy trinity in the genesis of colorectal cancer. Eur J Surg Oncol. 2003;29:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Hajra KM, Fearon ER. Cadherin and catenin alterations in human cancer. Genes Chromosomes Cancer. 2002;34:255-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 257] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 22. | Orford K, Orford CC, Byers SW. Exogenous expression of beta-catenin regulates contact inhibition, anchorage-independent growth, anoikis, and radiation-induced cell cycle arrest. J Cell Biol. 1999;146:855-868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 215] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, Kitajewski J, Wang CY. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J Cell Biol. 2001;152:87-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 333] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 24. | Gottardi CJ, Wong E, Gumbiner BM. E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. J Cell Biol. 2001;153:1049-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 436] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 25. | Watabe M, Hishikawa K, Takayanagi A, Shimizu N, Nakaki T. Caffeic acid phenethyl ester induces apoptosis by inhibition of NFkappaB and activation of Fas in human breast cancer MCF-7 cells. J Biol Chem. 2004;279:6017-6026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 170] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Lee YJ, Kuo HC, Chu CY, Wang CJ, Lin WC, Tseng TH. Involvement of tumor suppressor protein p53 and p38 MAPK in caffeic acid phenethyl ester-induced apoptosis of C6 glioma cells. Biochem Pharmacol. 2003;66:2281-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |