Published online Jul 14, 2005. doi: 10.3748/wjg.v11.i26.3998
Revised: October 2, 2004
Accepted: October 7, 2004
Published online: July 14, 2005
AIM: To ascertain the adequacy of the microsatellite instability (MSI) as a prognostic indicator by assessing MSI status of patients with double primary gastric and colorectal cancer (DPGCC).
METHODS: Sixteen patients were studied, all of whom exhibited sporadic DPGCC, and had no family history of hereditary non-polyposis colorectal cancer, according to the Amsterdam criteria. A total of 32 cancers from 16 DPGCC patients, and 216 single primary CRC, were assessed for MSI in 5 microsatellite loci, BAT25, BAT26, D2S123, D5S346, and D17S250.
RESULTS: MSI was observed in 6 (37.5%) of 16 GC and 4 (25.0%) of 16 CRC. Thirty tumors (13.9%) out of 216 single primary CRC and one tumor (16.7%) out of 6 double primary CRC were found to be microsatellite unstable. Of the 6 GC with MSI in DPGCC, 5 (31.3%) were MSI-high and one (6.3%) was MSI-low. In 5 of 16 DPGCC patients, the cancer recurred in or adjacent to the anastomosis or metastasized to the kidney or lung. The MSI-high DPGCC cases were associated with a younger age of onset (47.5 years vs 62.5 years), higher frequency of lymph node metastasis (100% vs 25%), and advanced Dukes stage (C, 100% vs 41.7%), as well as a higher frequency of recurrence or metastasis (100% vs 8.3%). Only recurrence or metastasis showed statistical significance by Fisher’s exact test.
CONCLUSION: Our data suggest that MSI may play an important role in the development of DPGCC, and that it may be used clinically as a molecular predictive marker for recurrence or late metastasis of DPGCC.
- Citation: Kim YH, Song SY, Kwon YD, Kim DS, Chun HK, Rhee JC. Microsatellite instable double primary cancers of the colorectum and stomach exhibit less favorable outcome. World J Gastroenterol 2005; 11(26): 3998-4002
- URL: https://www.wjgnet.com/1007-9327/full/v11/i26/3998.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i26.3998
Gastric cancers (GC) and colorectal cancers (CRC) are common neoplasms in Korea and Japan. Almost 5% of GC and CRC patients develop other primary gastrointestinal (GI) cancers, either synchronously or metachronously[1]. GC is the most prevalent extra CRC among multiple primary GI cancers involving CRC in Korea and Japan[2,3]. The determination of high-risk groups of patients with multiple primary cancers involving the GI tract is important for clinical management, especially with regard to the prediction of patient’s prognoses[4]. The most well-known example of multiple primary cancers is hereditary non-polyposis colorectal cancer (HNPCC), an autosomally dominant hereditary syndrome which is caused by germline mutations in mismatch repair genes, including hMSH2, hMLH1, hPMS1, hPMS2, and hMSH6. Ninety percent of HNPCCs exhibit microsatellite instability (MSI)[5]. MSI has also been observed in approximately 15-30% of single sporadic GC[6,7] and in 10-15% of single sporadic CRC[8,9]. Germline mutations of MLH1 and MSH2 occur at approximately equal frequencies in HNPCC, whereas virtually all sporadic MSI-high cancers develop due to epigenetic silencing of the MLH1 promoter through somatic hypermethylation[10-13]. MSI may impart a favorable prognosis in colorectal, gastric, pancreatic, and probably esophageal cancers, but a poor prognosis in non-small cell lung cancer[14].
Some reports have suggested the contribution of MSI in double primary cancers involving both the colorectum and stomach in the same patient[4,15-17]. According to these reports, MSI is an indicator of increased cancer susceptibility in these double primary gastric and colorectal cancer (DPGCC) patients and genetic instability may play an important role in the development of these cancers. However, little remains known regarding the prognostic role of MSI in DPGCC, although a number of previous studies have focused on the etiology or carcinogenic mechanisms of DPGCC. Therefore, we examined MSI status in patients with DPGCC in order to ascertain the adequacy of MSI as a prognostic indicator.
Sixteen patients with sporadic DPGCC were studied. None of these cases had any family history of the HNPCC, according to the Amsterdam criteria for HNPCC. All patients underwent curative resections of both stomach and large bowel with tumor-free resection margins at the Department of Surgery, Samsung Medical Center, between January, 1994 and December, 2002, with the exception of one (case 12). Clinicopathological features are summarized in Table 1. Ages ranged from 35 to 76 (58.8 ± 10.1) years. Patients with synchronous DPGCC were defined as cases diagnosed with a secondary cancer that was detected within 6 mo of the detection of primary cancer, and those with metachronous DPGCC were defined as cases diagnosed with a secondary cancer detected more than 6 mo later, as previously described[15,18]. Our study included 12 cases of synchronous (75.0%) and 4 cases of metachronous (25.0%) DPGCC. In one out of the 4 metachronous cases, the initial cancer was GC; in the remaining 3, it was CRC. The average ages of patients with synchronous and metachronous DPGCC were the same as the mean, 58.8 years. In cases of GC, 10 were found to be intestinal type (62.5%), and 6 cases were diffuse type (37.5%). Four cases were early type (25.0%), and 12 cases were advanced type (75.0%). No lymph node metastasis was detected in any of the cases of GC, and all cases were at stage I. In cases of CRC, six (37.5%) were located at the left-side of the colon or in the rectum. Four cases exhibited mucinous differentiation (25.0%) and 12 cases evidenced moderate tubular differentiation (75.0%). Nine cases (56.3%) of CRC exhibited lymph node metastasis, and 14 (87.5%) were at an advanced stage (Dukes B or C).
| Case | Sex | Age (yr) | Onset | Gastric cancer | Site | Colorectal cancer | ||||||
| Differentiation | T | N | Stage | Differentiation | T | N | Stage | |||||
| 1 | Male | 49 | Synchronous | Diffuse | 1 | 0 | 1 | TC | Moderate | 4 | 1 | 3/C |
| 2 | Male | 35 | Synchronous | Intestinal | 2 | 0 | 1 | AC | Mucinous | 3 | 2 | 3/C |
| 3 | Male | 50 | Metachronous | Intestinal | 2 | 0 | 1 | DC | Moderate | 3 | 1 | 3/C |
| 4 | Male | 56 | Synchronous | Intestinal | 1 | 0 | 1 | C | Moderate | 3 | 1 | 3/C |
| 5 | Male | 68 | Synchronous | Intestinal | 1 | 0 | 1 | SC | Mucinous | 3 | 0 | 2/B |
| 6 | Male | 57 | Synchronous | Diffuse | 1 | 0 | 1 | R | Moderate | 3 | 2 | 3/C |
| 7 | Male | 54 | Synchronous | Intestinal | 2 | 0 | 1 | SC | Moderate | 2 | 0 | 1/A |
| 8 | Male | 61 | Synchronous | Intestinal | 1 | 0 | 1 | AC | Moderate | 2 | 0 | 1/A |
| 9 | Female | 76 | Metachronous | Intestinal | 1 | 0 | 1 | TC | Moderate | 3 | 0 | 2/B |
| 10 | Male | 65 | Synchronous | Intestinal | 1 | 0 | 1 | AC | Moderate | 3 | 0 | 2/B |
| 11 | Female | 65 | Synchronous | Diffuse | 1 | 0 | 1 | AC | Mucinous | 3 | 1 | 3/C |
| 12 | Male | 48 | Metachronous | Diffuse | ? | ? | ? | AC | Moderate | 3 | 2 | 3/C |
| 13 | Male | 71 | Synchronous | Intestinal | 1 | 0 | 1 | AC | Well | 3 | 1 | 3/C |
| 14 | Male | 65 | Synchronous | Diffuse | 1 | 0 | 1 | SC | Moderate | 3 | 0 | 2/B |
| 15 | Male | 59 | Synchronous | Intestinal | 1 | 0 | 1 | AC | Mucinous | 3 | 0 | 2/B |
| 16 | Male | 61 | Metachronous | Diffuse | 2 | 0 | 1 | SC | Moderate | 3 | 1 | 3/C |
Two hundred and sixteen single primary CRC, and 6 double primary CRC, were analyzed for MSI for the comparison in the current study for DPGCC.
We followed the recurrence and survival of 16 patients with DPGCC for at least 18 mo.
Microdissection was performed by an expert pathologist in this field using 10-µm-thick hematoxylin-stained tissue sections in order to reduce contamination from other cellular DNA components. DNA was extracted from formalin-fixed, paraffin-embedded archival specimens of tumors and matched normal tissues, using the DNeasy Tissue kit (Qiagen, Hilden, Germany).
Five primers from independent genomic sites, including two mononucleotide repeat microsatellites (BAT25 and BAT26) and three dinucleotide repeat microsatellites (D2S123, D5S346 and D17S250), all of which were recommended by the National Cancer Institute workshop, were used in this study. Forward primers were synthesized with a fluorescent tag (FAM and NED) on the 5’ end, and were purified by standard high-performance liquid chromatography. One hundred nanograms of DNA was amplified in a 20 µL reaction solution, which containing 2 µL of 10× buffer (Roche, Mannheim, Germany), 1.75-3 mmol/L MgCl2, 0.4 µmol/L primer pairs, 250 µmol/L deoxynucleoside triphosphate, and 2.5 units of DNA polymerase (Roche, Mannheim, Germany). Amplifications were performed using a 2 min initial denaturation at 94°C, followed by 30 cycles of 45s at 94°C, 45s at 55°C, and 45s at 72°C, and 5 min final extension at 72°C. The fluorescence-labeled PCR products were electrophoresed on an Applied Biosystems 3100 automated DNA sequencer (Applied Biosystems, Foster, USA), and the fluorescent signals from the differently-sized alleles were recorded and analyzed using Genescan software (version 2.7; Applied Biosystems).
Either Fisher’s exact tests or Mann-Whitney tests were used to analyze variations in MSI status between various parameters. P values of less than 0.05 were considered statistically significant. Specialized statistical assistance was warranted by the small number of samples.
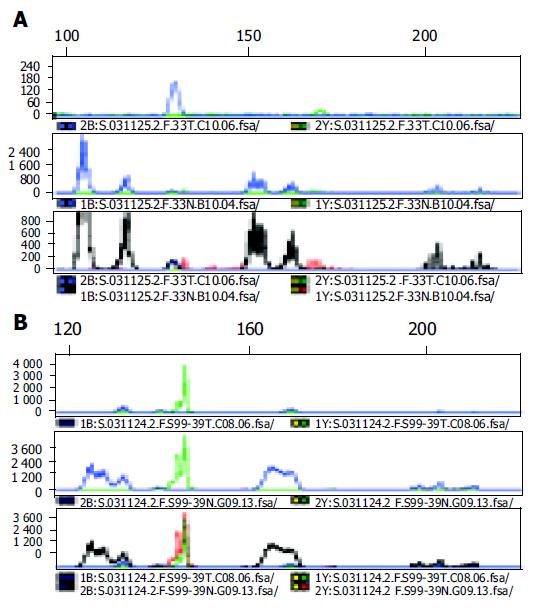
A total of 32 cancers from 16 Korean DPGCC patients and 216 single primary CRC cases were assessed for MSI, for 5 microsatellite loci. MSI results are summarized in Table 2. MSI was observed in six (37.5%) of 16 GC and 4 (25.0%) of 16 CRC in DPGCC patients (Figure 1). Thirty (13.9%) tumors of 216 single primary CRCs and one (16.7%) tumor of 6 double primary CRCs were found to be microsatellite unstable. Of the 6 GC with MSI in DPGCC, 5 (31.3%) were MSI-high and one (6.3%) was MSI-low. All CRC with MSI were MSI-high. There was an increasing tendency of MSI detected, with the lowest probability in single primary CRC, followed by double primary CRC, CRC of DPGCC, and GC of DPGCC. Two of six patients with MSI (33.3%) exhibited heterogeneity in terms of microsatellite alterations. For example, one lesion exhibited the MSI phenotype, but the other lesion in the same patient proved uninformative. One MSI in double primary CRC showed homogeneity with regard to MSI-high status.
| Case | Gastric cancer (GC) | Colorectal cancer (CRC) | Follow-up (mo) | ||||||||||
| bat26 | D5S346 | bat25 | D17S250 | D2S123 | MSI | bat26 | D5S346 | bat25 | D17S250 | D2S123 | MSI | ||
| 1 | ● | ● | ● | H | ● | ● | ⊙ | H | 21, alive, RGC | ||||
| 22, alive, RCRC | |||||||||||||
| 2 | ● | ● | ● | ⊙ | ● | H | ● | ● | ⊙ | ⊙ | ⊙ | H | 88, alive, MGC, kidney |
| 3 | ● | ● | ● | ⊙ | ● | H | ● | ○ | ● | ○ | ● | H | 58, alive, +AOV cancer |
| 4 | ● | ○ | ● | ○ | ○ | H | ● | ○ | ● | ○ | ○ | H | 10, alive, RCRC |
| 5 | ● | ● | ● | ● | ● | H | ● | ○ | ⊙ | ⊙ | ⊙ | I | 18, alive, NED |
| 6 | ○ | ○ | ● | ○ | ○ | L | ⊙ | ⊙ | ⊙ | ⊙ | ⊙ | I | 38, alive, NED |
| 7 | ○ | ○ | ○ | ○ | ○ | S | ○ | ○ | ○ | ○ | ○ | S | 29, alive, NED |
| 8 | ○ | ○ | ○ | ○ | ○ | S | ○ | ○ | ○ | ○ | ○ | S | 18, alive, NED |
| 9 | ○ | ○ | ○ | ○ | ○ | S | ○ | ○ | ○ | ○ | ○ | S | 114, alive, NED |
| 10 | ○ | ○ | ○ | ○ | ○ | S | ○ | ○ | ○ | ○ | ○ | S | 85, alive, NED |
| 11 | ○ | ○ | ○ | ○ | ○ | S | ○ | ○ | ○ | ○ | ○ | S | 61, alive, NED |
| 12 | ○ | ○ | ○ | ○ | ○ | S | ○ | ○ | ○ | ○ | ○ | S | 82, alive, NED |
| 13 | ⊙ | ⊙ | ⊙ | ⊙ | ⊙ | I | ○ | ○ | ○ | ○ | ○ | S | 19, alive, NED |
| 14 | ○ | ○ | ○ | ○ | ○ | S | ⊙ | ⊙ | ⊙ | ⊙ | ⊙ | I | 17, alive, NED |
| 15 | ⊙ | ○ | ⊙ | ⊙ | ⊙ | I | ○ | ○ | ○ | ○ | ○ | S | 29, alive, NED |
| 16 | ⊙ | ○ | ⊙ | ⊙ | ⊙ | I | ○ | ○ | ○ | ○ | ○ | S | 67, alive, MCRC, lung |
Five patients of 16 DPGCC exhibited recurrence, either in or adjacent to the anastomosis or metastasis to different organs, such as the kidney or lung (Table 2). Case 1 exhibited recurrence of GC, 21 mo after the initial diagnosis, and recurrence of CRC 22 mo, after the initial diagnosis, both occurring at the anastomosis site. Case 2 exhibited metastatic GC in the right kidney, 88 mo after initial diagnosis. Case 3 exhibited an ampulla of Vater cancer at the time of initial diagnosis. Case 4 exhibited a recurrent CRC in another colonic segement (initial, cecum; recurred, transverse colon) 10 mo after the initial diagnosis. Case 16 exhibited metastatic CRC in the left lung 67 mo after the initial diagnosis.
The MSI-high DPGCC was associated with a younger age of onset (47.5 years vs 62.5 years), a higher frequency of lymph node metastasis (100% vs 25%), advanced Dukes stage (C, 100% vs 41.7%), and higher frequency of recurrence or metastasis (100% vs 8.3%) (Table 3). Only recurrence or metastasis showed statistical significance, with P values of less than 0.005, by Fisher’s exact test.
| Both MSI-high (n = 4) | Others (n = 12) | P | |
| Male : Female | 4:00 | 10:02 | NS |
| Age (yr) | 47.5 ± 8.9 (35-56) | 62.5 ± 7.6 (48-76) | NS |
| Gastric cancer | |||
| - intestinal : diffuse | 3:01 | 7:05 | NS |
| - size (cm) | 2.8 | 3.1 | NS |
| Colorectal cancer | |||
| - tubular : mucinous | 3 : 1 | 9 : 3 | NS |
| - size (cm) | 4.7 | 4.2 | NS |
| - node metastasis | 100% | 25% | NS |
| - Dukes A : B : C | 0 : 0 : 4 | 2 : 5 : 5 | NS |
| Recurrence, metastasis, | 100% | 8.3% | < 0.005 |
| or other organ malignancy |
MSI has been suggested to play an important role in the development of multiple primary cancers of the gastrointestinal tract. Thus, MSI appears to be useful in screening for the detection of a high-risk group for secondary primary cancer in some patients with sporadic single gastric and colorectal cancers, because it is rapid and inexpensive.
MSI has been observed in approximately 15-30% of single sporadic gastric cancers[6,7] and 10-15% of single sporadic colorectal cancers[8,9]. Our results regarding MSI rate in double primary CRC and single sporadic CRC, were not radically different from the results of other investigators. In our study, MSI was observed in 31.3%, 25.0%, 16.7%, and 13.9% of GC in patients with DPGCC, CRC of patients with DPGCC, double primary CRC, and single primary CRC, respectively. This indicates that genetic instability may play an important role in the development of multiple primary cancers of the GI tract. MSI has been observed more frequently in multiple GI cancer patients than in those with single primary gastric or colorectal cancers, as demonstrated in previous reports[3,4]. Concordant MSI rate, and MSI-high status in both cancers, has been reported 17.7-25.0% of DPGCC[3,15]. Our result is very similar to these. Yamashita et al[4] reported that MSI-high status was seen more frequently in the same organ group, especially in multiple GC patients, than in different organ groups, and lesions from different organ groups tended to show MSI-low or stable phenotype. Thus, MSI in multiple GI cancers containing GC appears to be relatively common, and may play an important role in multi-organ carcinogenesis.
The relationship between MSI of DPGCC and patient prognosis or clinicopathological features remains unclear. Kim et al[3] reported that MSI-positive CRC was characterized by fungating tumor gross, poor or mucinous differentiation, and rare nodal metastasis. MSI-positive GC was characterized by intestinal type, smaller size, and rare nodal spread. They were unable to explain discrepancies in size and differentiation between CRC and GC. According to our result, the clinicopathological features of GC were basically the same concordantly MSI-positive GC as was observed in other cases of DPGCC. Studied cases were usually intestinal type, and at a relatively early stage. Differences, in general, were seen in the age and the clinicopathological features of CRC. Concordantly MSI-positive CRC was associated with younger age, larger tumor size, more frequent nodal metastasis, higher stage, and more frequent recurrence and distant metastasis. Our results were also different from others. Ohtani et al[15] reported that concordantly MSI-positive CRC was usually associated with better prognosis. There were no dead patients found, among 5 concordantly MSI-positive DPGCC, and 4 dead patients (26.7%) among the 15 remaining cases. However, no explanation was given in their report regarding about the causes of death. In our results, we found no cancer-related or natural deaths in the studied cases. Ikeda et al[19] compared the prognosis of patients with DPGCC with patients with single cancer, and reported that the former was better than the latter. One explanation for this difference in prognosis may be that DPGCC is often discovered at an early stage. According to our results, the stage of DPGCC was more advanced in concordantly MSI-positive DPGCC. Thus, we suggested that the prognosis of patients with DPGCC appears to be dependent on stage.
Interestingly, there have been no reports regarding the recurrence or late metastasis in patients with DPGCC. We followed the hospital course of all studied cases. Case 1 was a unique example of cancer recurrence in patients with concordantly MSI-positive DPGCC. This patient had mucosa-confined early gastric cancer and Dukes C CRC in the transverse colon synchronously, and underwent curative resection for both organs with ample resection margins. However, both tumors recurred at the anastomosis sites, 21 and 22 mo after the initial diagnosis. Case 2 was also very unique. He had submucosa-confined early gastric cancer and Dukes C CRC in the ascending colon synchronously, and underwent curative resection for both organs with ample resection margins. The patient then had an uneventful history for 7 years. However, renal metastasis of previous early gastric cancer was found 88 mo after the initial diagnosis. Cases 4 and 16 were examples of recurrence and late distant metastasis of advanced-stage CRC. Recent studies have revealed the significance of MSI in gastric carcinogenesis. Kashiwagi et al[20] reported that MSI in the gastric biopsy specimens of chronic gastritis patients may predict the risk of progression to adenoma and well-differentiated adenocarcinoma. Kim et al[21] reported that gastric carcinomas arising from adenomas are frequently associated with MSI. These data, and ours, suggest that MSI might play an important role in the early phase of carcinogenesis, and is, therefore, related to the delayed development of recurrent disease. Less clear is the role of and mechanisms underlying MSI-low status. In our study, only one MSI-low was found.
Due to its rarity, the meaning of MSI in DPGCC, whether concordantly-positive or not, is still a topic which warrants future investigation. Our data suggest that MSI may play an important role in the development of DPGCC, and that it may be used clinically as a molecular marker for the prediction of recurrence or late metastasis of DPGCC.
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Tomoda H, Taketomi A, Baba H, Kohnoe S, Seo Y, Saito T. Multiple primary colorectal and gastric carcinoma in Japan. Oncol Rep. 1998;5:147-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Akagi Y, Araki Y, Ogata Y, Morodomi T, Shirouzu K, Isomoto H, Kakegawa T. Clinicopathological studies of multiple cancers in the large bowel and other organs. Kurume Med J. 1993;40:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Kim HC, Kim CN, Jung CS, Yu CS, Kim JC. Multiple primary malignant neoplasm with colorectal cancer. J Korean Cancer Assoc. 1998;30:668-674. |
| 4. | Yamashita K, Arimura Y, Kurokawa S, Itoh F, Endo T, Hirata K, Imamura A, Kondo M, Sato T, Imai K. Microsatellite instability in patients with multiple primary cancers of the gastrointestinal tract. Gut. 2000;46:790-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Liu B, Parsons R, Papadopoulos N, Nicolaides NC, Lynch HT, Watson P, Jass JR, Dunlop M, Wyllie A, Peltomäki P. Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients. Nat Med. 1996;2:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 599] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 6. | Strickler JG, Zheng J, Shu Q, Burgart LJ, Alberts SR, Shibata D. p53 mutations and microsatellite instability in sporadic gastric cancer: when guardians fail. Cancer Res. 1994;54:4750-4755. [PubMed] |
| 7. | Chong JM, Fukayama M, Hayashi Y, Takizawa T, Koike M, Konishi M, Kikuchi-Yanoshita R, Miyaki M. Microsatellite instability in the progression of gastric carcinoma. Cancer Res. 1994;54:4595-4597. [PubMed] |
| 8. | Brown SR, Finan PJ, Hall NR, Bishop DT. Incidence of DNA replication errors in patients with multiple primary cancers. Dis Colon Rectum. 1998;41:765-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Aaltonen LA, Peltomäki P, Mecklin JP, Järvinen H, Jass JR, Green JS, Lynch HT, Watson P, Tallqvist G, Juhola M. Replication errors in benign and malignant tumors from hereditary nonpolyposis colorectal cancer patients. Cancer Res. 1994;54:1645-1648. [PubMed] |
| 10. | Thibodeau SN, French AJ, Cunningham JM, Tester D, Burgart LJ, Roche PC, McDonnell SK, Schaid DJ, Vockley CW, Michels VV. Microsatellite instability in colorectal cancer: different mutator phenotypes and the principal involvement of hMLH1. Cancer Res. 1998;58:1713-1718. [PubMed] |
| 11. | Esteller M, Levine R, Baylin SB, Ellenson LH, Herman JG. MLH1 promoter hypermethylation is associated with the microsatellite instability phenotype in sporadic endometrial carcinomas. Oncogene. 1998;17:2413-2417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 323] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Kane MF, Loda M, Gaida GM, Lipman J, Mishra R, Goldman H, Jessup JM, Kolodner R. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997;57:808-811. [PubMed] |
| 13. | Young J, Simms LA, Biden KG, Wynter C, Whitehall V, Karamatic R, George J, Goldblatt J, Walpole I, Robin SA. Features of colorectal cancers with high-level microsatellite instability occurring in familial and sporadic settings: parallel pathways of tumorigenesis. Am J Pathol. 2001;159:2107-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 278] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Lawes DA, SenGupta S, Boulos PB. The clinical importance and prognostic implications of microsatellite instability in sporadic cancer. Eur J Surg Oncol. 2003;29:201-212. [RCA] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Ohtani H, Yashiro M, Onoda N, Nishioka N, Kato Y, Yamamoto S, Fukushima S, Hirakawa-Ys Chung K. Synchro-nous multiple primary gastrointestinal cancer exhibits fre-quent microsatellite instability. Int J Cancer. 2000;86:678-683. [DOI] [Full Text] |
| 16. | Kim HS, Cho NB, Yoo JH, Shin KH, Park JG, Kim YI, Kim WH. Microsatellite instability in double primary cancers of the colorectum and stomach. Mod Pathol. 2001;14:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Ericson K, Halvarsson B, Nagel J, Rambech E, Planck M, Piotrowska Z, Olsson H, Nilbert M. Defective mismatch-repair in patients with multiple primary tumours including colorectal cancer. Eur J Cancer. 2003;39:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Lyons MF, Redmond J, Covelli H. Multiple primary neoplasia of the head and neck and lung. The changing histopathology. Cancer. 1986;57:2193-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Ikeda Y, Mori M, Kajiyama K, Haraguchi Y, Sugimachi K. Multiple primary gastric and colorectal cancer in Japan. Int Surg. 1995;80:37-40. [PubMed] |
| 20. | Kashiwagi K, Watanabe M, Ezaki T, Kanai T, Ishii H, Mukai M, Hibi T. Clinical usefulness of microsatellite instability for the prediction of gastric adenoma or adenocarcinoma in patients with chronic gastritis. Br J Cancer. 2000;82:1814-1818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Kim HS, Woo DK, Bae SI, Kim YI, Kim WH. Microsatellite instability in the adenoma-carcinoma sequence of the stomach. Lab Invest. 2000;80:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |