Published online Jul 14, 2005. doi: 10.3748/wjg.v11.i26.3980
Revised: August 23, 2004
Accepted: August 30, 2004
Published online: July 14, 2005
AIM: To study the in vitro and in vivo antitumor effect of lidamycin (LDM) on hepatoma and the active moiety of its molecule.
METHODS: MTT assay was used to determine the growth inhibition of human hepatoma BEL-7402 cells, SMMC-7721 cells and mouse hepatoma H22 cells. The in vivo therapeutic effects of lidamycin and mitomycin C were determined by transplantable hepatoma 22 (H22) in mice and human hepatoma BEL-7402 xenografts in athymic mice.
RESULTS: In terms of IC50 values, the cytotoxicity of LDM was 10 000-fold more potent than that of mitomycin C (MMC) and adriamycin (ADM) in human hepatoma BEL-7402 cells and SMMC-7721 cells. LDM molecule consists of two moieties, an aproprotein (LDP) and an enediyne chromophore (LDC). In terms of IC50 values, the potency of LDC was similar to LDM. However, LDP was 105-fold less potent than LDM and LDC to hepatoma cells. For mouse hepatoma H22 cells, the IC50 value of LDM was 0.025 nmol/L. Given by single intravenous injection at doses of 0.1, 0.05 and 0.025 mg/kg, LDM markedly suppressed the growth of hepatoma 22 in mice by 84.7%, 71.6% and 61.8%, respectively. The therapeutic indexes (TI) of LDM and MMC were 15 and 2.5, respectively. By 2 iv. injections in two experiments, the growth inhibition rates by LDM at doses of 0.1, 0.05, 0.025, 0.00625 and 0.0125 mg/kg were 88.8-89.5%, 81.1-82.5%, 71.2-74.9%, 52.3-59.575%, and 33.3-48.3%, respectively. In comparison, MMC at doses of 5, 2.5, and 1.25 mg/kg inhibited tumor growth by 69.7-73.6%, 54.0-56.5%, and 31.5-52.2%, respectively. Moreover, in human hepatoma BEL-7402 xenografts, the growth inhibition rates by LDM at doses of 0.05 mg/kg × 2 and 0.025 mg/kg × 2 were 68.7% and 27.2%, respectively. However, MMC at the dose of 1.25 mg/kg × 2 showed an inhibition rate of 34.5%. The inhibition rate of tumor growth by LDM was higher than that by MMC at the tolerated dose.
CONCLUSION: Both LDM and its chromophore LDC display extremely potent cytotoxicity to hepatoma cells. LDM shows a remarkable therapeutic efficacy against murine and human hepatomas in vivo.
- Citation: Huang YH, Shang BY, Zhen YS. Antitumor efficacy of lidamycin on hepatoma and active moiety of its molecule. World J Gastroenterol 2005; 11(26): 3980-3984
- URL: https://www.wjgnet.com/1007-9327/full/v11/i26/3980.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i26.3980
Lidamycin (LDM, also called C-1027), a macromolecular antitumor antibiotic produced by Streptomyces globisporus C-1027, can markedly inhibit the growth of transplantable tumors in mice including leukemia L1210, P388, sarcoma 180 and melanoma Harding-Passey[1-4]. LDM consists of an apoprotein (LDP) of 10.5 ku and an enediyne chromophore (LDC) of 843 ku. These two parts of the molecule, connecting each other through non-covalent binding, can be dissociated[5]. LDM can induce DNA damages including double-strand breaks, single-strand breaks and formation of abasic sites which is proved to be the major mechanism of the cytotoxicity of LDM. It is also reported that LDC can interact in DNA minor grooves and cleave double-helical DNA with a remarkable sequence-selectivity. The double-strand cleavage sites occurring predominantly at CTTTT/AAAAG, ATAAT/ATTAT, CTTTA/TAAAG, CTCTT/AAGAG, and especially GTTAT/ATAAC, consist of nucleotide sequences with a two-nucleotide 3’-stagger of the cleaved residues[6].
Recently, LDM has entered phase I clinical trials. In this study, we report the growth inhibition of various carcinoma cell lines by LDM, and the therapeutic efficacy of LDM against hepatoma in mice and human hepatoma xenografts in athymic mice.
Highly purified LDM and LDP were prepared in our institute. MTT was obtained from Sigma Chemical Co. (St. Louis, MO, USA). Mitomycin C (MMC) was purchased from Kyowa Hakko Kogyo Co. Ltd. Adriamycin (ADM) was purchased from Zhejiang Hisun Pharmaceutical Co. Ltd., China.
Human hepatoma BEL-7402 cells, SMMC-7721 cells and murine hepatoma 22 cells were cultured at 37°C with 50 ml/L CO2 in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, penicillin G (100 U/mL) and streptomycin (100 µg/mL). All cell lines were passaged every 3 d and maintained in exponential growth to approximately 80% confluence for experiments.
After 10 mg LDM was mixed with 5 mL methanol for 5 min at 4°C, the mixture was placed at -20°C and tossed for one time. LDC in the supernatant of reaction mixture was obtained by centrifugation of 16000 g for 20 min at 4°C. In order to extract LDC entirely, the operation was repeated three times.
Cells were detached by trypsinization, seeded at 3000 cells/ well in a 96-well plate (Costar, Cambridge, MA) over night. Then different concentrations of LDM were added and incubated for an additional 48 h. The effect on cell growth was examined by MTT assay. Briefly, 20 µL of MTT solution (5 mg/mL) was added to each well and incubated at 37°C for 4 h. The supernatant was removed, and the MTT formazan formed by metabolically viable cells was dissolved in 150 µL of DMSO, and then monitored with a microplate reader (Bio-Rad) at a wavelength of 560 nm. Survival ratio was calculated according to the following formula:
Survival ratio = (Atest-Ablank)/(Acontrol-Ablank) × 100%
Female Kunming mice weighting 20 ± 2 g and BALB/c (nu/nu) athymic mice were obtained from the Institute of Experimental Animals, Chinese Academy of Medical Sciences (CAMS) and Peking Uinion Medical College (PUMC).
Therapeutic effects of LDM and MMC were assessed using murine hepatoma 22 models. Groups of 10 Kunming female mice were subcutaneously implanted with 1.5 × 106 tumor cells. For experiment I, the drugs were administrated 24 h after tumor transplantation with single intravenous injection. For experiments I and II, the drugs were iv administrated 24 h and 7 d after tumor transplantation. PBS was used as control in each experiment. Animals were sacrificed on day 11 and tumor weights (W) were measured. Inhibition rate of tumor growth and therapeutic index were calculated respectively as follows:
Inhibition (%) = [(W control-W treated)/W control] × 100 Therapeutic index (TI) = LD5/ID50
Therapeutic effect of LDM on human hepatoma BEL-7402 xenografts in athymic mice was assessed. Groups of six athymic female mice were subcutaneously implanted with hepatoma BEL-7402 tumor tissue fragments, 2 mm in diameter, one for each mouse. LDM and MMC in PBS were administrated iv on d 3 and 10 after tumor implantation. PBS was used as control. Tumor volume (V) was measured twice a week using a caliper and calculated using the formula: V (mm3) =1/2ab2, where a and b represent the long diameter and perpendicular short diameter (mm) of the tumor, respectively.
Significant difference between two values was determined with Student’s t-test. P < 0.05 was considered statistically significant.
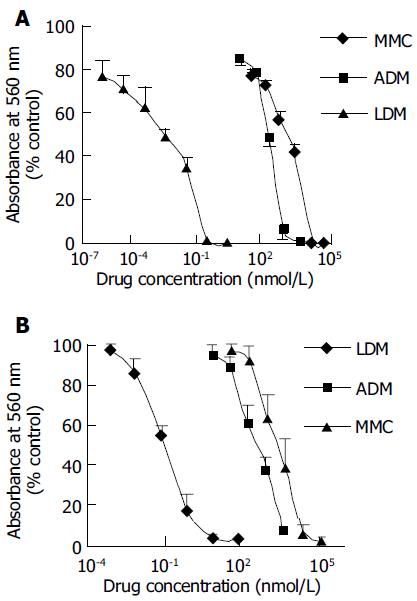
As shown in Figures 1A and B, LDM displayed extremely potent cytotoxicity to hepatoma BEL-7402 and SMMC-7721 cells. In comparison, the cytotoxicity of LDM was much more potent than that of MMC and ADM. The IC50 value of LDM, MMC and ADM was 0.0030, 152, and 51.7 nmol/L for BEL-7402 cells respectively, and 0.064, 1136.6, and 378 nmol/L for SMMC-7721 cells respectively. In terms of IC50 values, the cytotoxicity of LDM to hepatoma cells was 10 000-fold more potent than that of MMC and ADM (Table 1). In addition, the growth inhibitory IC50 value of LDM for mouse hepatoma 22 was 0.025 nmol/L by MTT assay (curve not shown).
| Drugs | IC50 (nmol/L) | |
| BEL-7402 | SMMC-7721 | |
| LDM | 0.003 ± 0.0006 | 0.064 ± 0.013 |
| MMC | 152.0 ± 27.3 | 1 136.6 ± 72.2 |
| ADM | 51.7 ± 6.5 | 378.0 ± 39.6 |
| LDC | 0.0032 ± 0.0005 | 0.0597 ± 0.0064 |
| LDP | 2 896.0 ± 345.5 | 1 896.0 ± 354.0 |
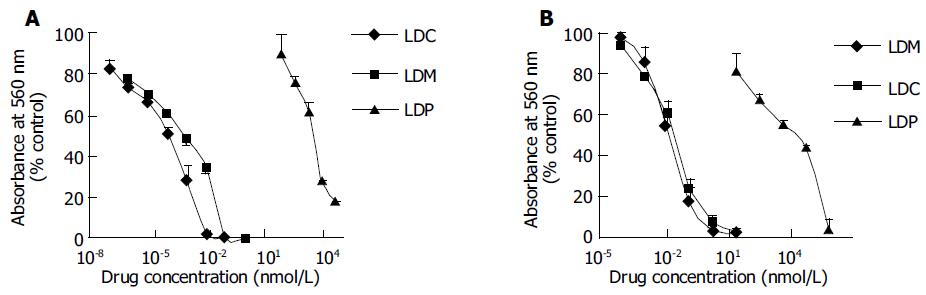
The inhibitory effects of LDM and its constituents, LDC and LDP, on cell proliferation were evaluated with MTT assay. As shown in Figures 2A and B, LDM and its constituent LDC displayed extremely potent cytotoxicity to human hepatoma BEL-7402 and SMMC-7721 cells. However, another constituent LDP was much less active to hepatoma cells. The IC50 value of LDC on BEL-7402 and SMMC-7721 cells was 0.0032 and 0.0597 nmol/L, respectively. However, the growth inhibitory IC50 value of LDP on BEL-7402 and SMMC-7721 cells was 2 896 and 1 896 nmol/L respectively. In terms of IC50 values, the potency of LDC was similar to LDM, but LDP was 105-fold less potent than LDM and LDC (Table 1).
The in vivo therapeutic efficacy of LDM and MMC on the growth of murine hepatoma 22 (H22) in Kunming mice was investigated. As shown in Table 2, LDM and MMC inhibited the growth of hepatoma 22 tumors in a dose dependent manner. In experiment I, LDM with single i.v.
| Experiment Groups | Dose(mg/kg) | Ivtimes | Number of mice | Body weight (g) | Tumorweight (g) | Inhibitionrate (%) | P | |||
| Begin | End | Begin | End | |||||||
| I | Control | 1 | 10 | 10 | 19.6 | 35 | 4.19 ± 1.36 | --- | --- | |
| LDM | 0.1 | 1 | 10 | 10 | 20.2 | 26.5 | 0.64 ± 0.17 | 84.7 | < 0.01 | |
| 0.05 | 1 | 10 | 10 | 19.6 | 27.5 | 1.19 ± 0.50 | 71.6 | < 0.01 | ||
| 0.025 | 1 | 10 | 10 | 19.9 | 30.3 | 1.60 ± 0.61 | 61.8 | < 0.01 | ||
| 0.0125 | 1 | 10 | 10 | 19.5 | 30.7 | 1.65 ± 0.50 | 60.6 | < 0.01 | ||
| 0.00625 | 1 | 10 | 10 | 19.9 | 31.5 | 2.48 ± 0.78 | 40.8 | < 0.01 | ||
| MMC | 5 | 1 | 10 | 10 | 19.8 | 32.3 | 1.72 ± 0.40 | 58.9 | < 0.01 | |
| 2.5 | 1 | 10 | 10 | 19.9 | 31.5 | 2.10 ± 0.84 | 49.9 | < 0.01 | ||
| 1.25 | 1 | 10 | 10 | 20 | 33.4 | 2.34 ± 0.83 | 44.2 | < 0.01 | ||
| II | Control | 2 | 10 | 10 | 20.2 | 35.2 | 3.83 ± 1.22 | --- | --- | |
| LDM | 0.1 | 2 | 10 | 10 | 20.2 | 23.5 | 0.43 ± 0.16 | 88.8 | < 0.01 | |
| 0.05 | 2 | 10 | 10 | 19.7 | 26.6 | 0.67 ± 0.20 | 82.5 | < 0.01 | ||
| 0.025 | 2 | 10 | 10 | 20.1 | 29.6 | 0.96 ± 0.30 | 74.9 | < 0.01 | ||
| 0.0125 | 2 | 10 | 10 | 20.3 | 32.8 | 1.55 ± 0.63 | 59.5 | < 0.01 | ||
| 0.00625 | 2 | 10 | 10 | 20 | 31.9 | 1.98 ± 0.77 | 48.3 | < 0.01 | ||
| MMC | 5 | 2 | 10 | 9 | 19.6 | 23.9 | 1.01 ± 0.29 | 73.6 | < 0.01 | |
| 2.5 | 2 | 10 | 10 | 20.2 | 30.2 | 1.76 ± 0.76 | 54 | < 0.01 | ||
| 1.25 | 2 | 10 | 10 | 20.3 | 33.8 | 1.83 ± 0.48 | 52.2 | < 0.01 | ||
| III | Control | 2 | 10 | 10 | 19.2 | 28.9 | 3.33 ± 1.04 | --- | --- | |
| LDM | 0.1 | 2 | 10 | 10 | 19.7 | 20.2 | 0.35 ± 0.06 | 89.5 | < 0.01 | |
| 0.05 | 2 | 10 | 10 | 19.8 | 25.2 | 0.63 ± 0.23 | 81.1 | < 0.01 | ||
| 0.025 | 2 | 10 | 10 | 19.2 | 23.7 | 0.96 ± 0.45 | 71.2 | < 0.01 | ||
| 0.0125 | 2 | 10 | 10 | 19.4 | 27.8 | 1.59 ± 0.72 | 52.3 | < 0.01 | ||
| 0.00625 | 2 | 10 | 10 | 19.6 | 26.6 | 2.22 ± 0.81 | 33.3 | < 0.05 | ||
| MMC | 5 | 2 | 10 | 10 | 19.8 | 25.1 | 1.08 ± 0.59 | 67.6 | < 0.01 | |
| 2.5 | 2 | 10 | 10 | 19.6 | 25.1 | 1.45 ± 0.88 | 56.5 | < 0.01 | ||
| 1.25 | 2 | 10 | 10 | 19.6 | 28.1 | 2.28 ± 0.96 | 31.5 | < 0.05 | ||
administration at the doses of 0.1, 0.05, 0.025, 0.0125, and 0.00625 mg/kg inhibited tumor growth by 84.7%, 71.6% and 61.8%, 60.6% and 40.8% (P < 0.01), respectively. Whereas, the inhibition rate of MMC at the doses of 5, 2.5, and 1.25 mg/kg was 58.9%, 49.9%, and 44.2%, respectively. The therapeutic index (TI) of LDM and MMC was 15 and 2.5, respectively. The therapeutic index of LDM was much higher than that of MMC. In experiments II and III, the drugs were administered on d 3 and 7. The growth inhibition rate by LDM was also higher than that of MMC. At doses used in these experiments, no body weight loss and other severe side-effects were observed, implying that LDM at tolerated doses displayed remarkable therapeutic efficacy.
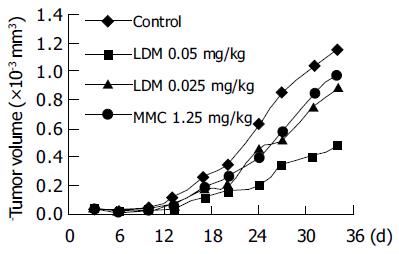
For further in vivo evaluation, the athymic mice bearing BEL-7402 hepatoma xenografts were treated by injection of LDM and MMC into the tail vein for the first time 3 d after subcutaneous tumor inoculation. At that time the tumor diameters ranged from 2 to 4 mm. Then the drugs were administered for the second time on d 10. As shown in Figure 3, LDM significantly inhibited the growth of BEL-7402 tumors. According to the tumor volume on d 24 (Table 3), the inhibition rate by LDM at the doses of 0.05 and 0.025 mg/kg was 68.7% (P < 0.05) and 27.2%, respectively. MMC at the dose of 1.25 mg/kg inhibited tumor growth by 34.5%. LDM showed much higher efficacy against the growth of hepatoma xenografts.
| Groups | Dose(mg/kg) | Number of mice(begin/end) | BWC (g) | Tumor volume(mm3) | Inhibitionrate (%) |
| Control | 6/6 | -0.1 | 624.7 ± 360.1 | - | |
| LDM | 0.05 | 6/6 | +0.3 | 195.6 ± 94.1 | 68.7a |
| 0.025 | 6/6 | -0.1 | 454.9 ± 302.3 | 27.2 | |
| MMC | 1.25 | 6/6 | -1.5 | 409.1 ± 225.9 | 34.5 |
Hepatoma progresses rapidly and has a poor prognosis. Although the cause is not fully understood, there are several known risk factors, including over 40 years of age, male sex, cirrhosis, and exposure to hepatitis viruses (hepatitis B, C, D and G), etc. Although surgical operation could cure some patients with hepatoma, many patients with carcinomas are not good surgical candidates because of large tumor size, diminished liver function, or cirrhosis. Therefore, it is urgent to develop new drugs for the treatment of carcinoma.
It was reported that LDM strongly inhibits DNA synthesis in hepatoma BEL-7402 cells. In terms of effective concentration, LDM is over 1 000-fold potent than MMC or ADM. LDM also inhibits RNA synthesis in hepatoma BEL-7402 cells without affecting protein synthesis, blocks BEL-7402 cells at G2/M phase[7] and induces mitotic cell death of human hepatoma BEL-7402 cells[8]. In the present study, LDM showed extreme cytotoxicity to hepatoma cells, including BEL-7402 SMMC-7721 and H22 cells in vitro. In our study, LDM displayed obvious tumor inhibition effect in vivo. The therapeutic efficacy of LDM was 6-fold higher than that of MMC. In addition, LDM showed potent anti-tumor effect on human hepatoma BEL-7402 xenografts in athymic mice in a dose dependent manner.
LDM has drawn much attention of researchers because of its potent anti-tumor effect, unique structure and action mechanism. Its apoprotein gene has been successfully cloned and nucleotide sequencing was determined[9]. Recently, as an example of enediyne anti-tumor antibiotics, the biosynthetic genes of LDM have become a hot spot[10]. In our studies on targeted anti-tumor drugs, LDM could act as a novel “effector moiety” to construct molecule-downsized and highly potent monoclonal antibody drugs. The downsized Fab’-LDM immunoconjugate is generated by chemical methods[11], which shows potent anti-tumor effect both in vitro and in vivo. We have demonstrated in this study that LDC has a similar potency as intact LDM molecule. In contrast, its apoprotein LDP displays weak effect on tumor cells. Recently, an energized fusion protein, Fv-LDP-AE, has been prepared. The highly potent fusion protein is composed of Fv fragment of antibody, protein moiety of LDM molecule, and the active enediyne (AE) of lidamycin[12]. The molecular weight of enediyne-energized fusion protein Fv-LDP-AE is only 38.7 ku, much smaller than the reported immunotoxin Fv-PE (67 ku). The method of preparing energized fusion protein Fv-LDP-AE may also provide a useful technical platform to construct scFv-based engineering and enediyne-energized fusion proteins specifically targeted to various cancers expressing different molecular markers.
In summary, both lidamycin and lidamycin chromophore have extremely potent cytotoxicity to liver cancer cells, and lidamycin shows remarkable therapeutic efficacy against murine transplantable hepatoma and human hepatoma xenografts.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Hu JL, Xue YC, Xie MY, Zhang R, Otani T, Minami Y, Yamada Y, Marunaka T. A new macromolecular antitumor antibiotic, C-1027. I. Discovery, taxonomy of producing organism, fermentation and biological activity. J Antibiot (Tokyo). 1988;41:1575-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Otani T, Minami Y, Marunaka T, Zhang R, Xie MY. A new macromolecular antitumor antibiotic, C-1027. II. Isolation and physico-chemical properties. J Antibiot (Tokyo). 1988;41:1580-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 56] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Zhen YS, Ming XY, Yu B, Otani T, Saito H, Yamada Y. A new macromolecular antitumor antibiotic, C-1027. III. Antitumor activity. J Antibiot (Tokyo). 1989;42:1294-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Zhen YS, Xue YC, Shao RG. Antitumor activity of the enediyne antibiotic C1027. Zhongguo Kangshengsu Zazhi. 1994;19:164-168. |
| 5. | Shao RG, Zhen YS. Relationship between the molecular composition of C-1027, a new macromolecular antibiotic with enediyne chromophore, and its antitumor activity. Yaoxue Xuebao. 1995;30:336-342. |
| 6. | Xu YJ, Zhen YS, Goldberg IH. C1027 chromophore, a potent new enediyne antitumor antibiotic, induces sequence-specific double-strand DNA cleavage. Biochemistry. 1994;33:5947-5954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Xu YJ, Li DD, Zhen YS. Mode of action of C-1027, a new macromolecular antitumor antibiotic with highly potent cytotoxicity, on human hepatoma BEL-7402 cells. Cancer Chemother Pharmacol. 1990;27:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | He QY, Liang YY, Wang DS, Li DD. Characteristics of mitotic cell death induced by enediyne antibiotic lidamycin in human epithelial tumor cells. Int J Oncol. 2002;20:261-266. [PubMed] |
| 9. | Sakata N, Ikeno S, Hori M, Hamada M, Otani T. Cloning and nucleotide sequencing of the antitumor antibiotic C-1027 apoprotein gene. Biosci Biotechnol Biochem. 1992;56:1592-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Liu W, Christenson SD, Standage S, Shen B. Biosynthesis of the enediyne antitumor antibiotic C-1027. Science. 2002;297:1170-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 224] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Liu XY, Zhen YS. [Antitumor effect of lidamycin-containing monoclonal antibody immunoconjugate with downsized-molecule]. Zhongguo YiXue KeXueYuan XueBao. 2001;23:563-567. [PubMed] |
| 12. | Li L, Huang YH, Miao QF, Shang BY, Liu XJ, Zhen YS. Fv- LDP-AE, an engineered and enedyine-energized fusion protein, shows highly potent antitumor efficacy and antiangiogenic activity. Proc Am Assoc Cancer Res. 2004;45:2884. |