Published online Jun 21, 2005. doi: 10.3748/wjg.v11.i23.3591
Revised: August 28, 2003
Accepted: September 23, 2003
Published online: June 21, 2005
AIM: To investigate if CD44v6 could be used as a molecular marker of cancer progression and metastasis through the detection of CD44v6 gene expression in normal human peripheral blood.
METHODS: RNA was extracted from the peripheral blood mononuclear cells of 50 healthy donors, the expression of CD44v6 was investigated using reverse transcriptase-polymerase chain reaction (RT-PCR).
RESULTS: CD44v6 mRNA was detected in 58% of healthy volunteers under the proper controls.
CONCLUSION: Our results suggest that the measurement of CD44v6 expression in peripheral blood by RT-PCR is not suitable for detection of circulating tumor cells.
-
Citation: Song J, Zhang DS, Zheng J. Expression of
CD44v6 gene in normal human peripheral blood. World J Gastroenterol 2005; 11(23): 3591-3594 - URL: https://www.wjgnet.com/1007-9327/full/v11/i23/3591.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i23.3591
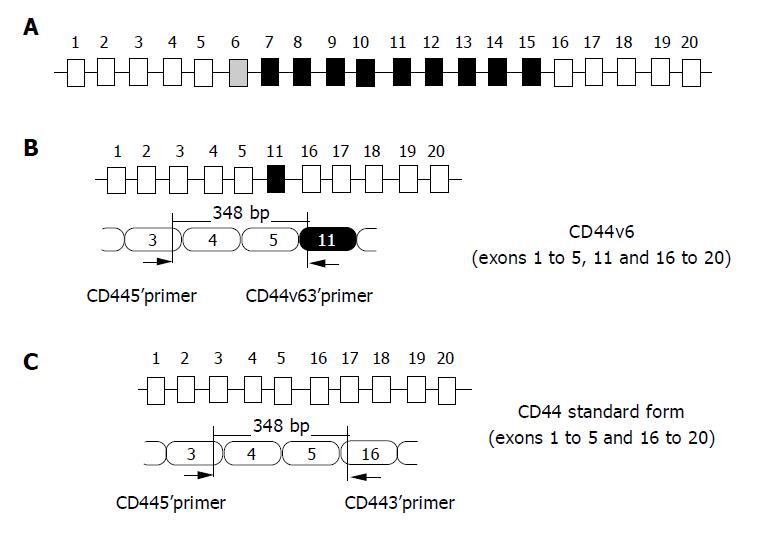
The main cause of cancer mortality is the formation of metastases in distant organs. Early detection of these tumor cells in peripheral blood is one of the most effective means of reducing cancer mortality. CD44 is a cell surface glycoprotein that regulates cell adhesion to elements of the extracellular matrix. Human CD44 gene on chromosome 1 lp13 that consists of 19 exons is divided into standard isoform (CD44s) and splicing variant isoform (CD44v) according to splicing patterns of its exons (Figure 1A and 1C)[1]. CD44s, the shortest isoform of CD44, is necessarily expressed on all cell types, whereas CD44v is selectively expressed on several normal cell types and some tumor cells.
In recent years, the role of CD44v6 has drawn attention to the invasion, metastasis and prognosis of tumors. Although several laboratories have reported that CD44v6 expression could be used as a potential marker of distant metastasis[2], other laboratories failed to confirm these results[3,4]. To determine whether CD44v6 expression could be used as a potential tumor marker of circulating tumor cells, we studied the CD44v6 expression in peripheral blood from 50 healthy volunteers using reverse transcriptase-polymerase chain reaction (RT-PCR) assay (Figure 1B).
Gastric carcinoma cell line MGC-803 expressing CD44v6[5] was grown in RPMI-1640 containing L-glutamine and sodium bicarbonate supplemented with 10% fetal calf serum, 100 IU/mL penicillin and 0.1 mg/mL streptomycin in a 50 mL/L CO2 incubator at 37 °C and cells were sub-cultured every 4 d.
Peripheral blood samples were obtained from healthy donors aged between 22 and 69 years. The first 10 mL blood was discarded to reduce contamination by epithelial cells at the site of needle entry. A further 5 mL was then collected in a silanized vacuum tube containing 0.6 mL 1.5% potassium ethylenediamine tetraacetic acid (EDTA) and promptly transported to the laboratory at 4 °C for at least 2 h before treatment. Peripheral blood nucleated cells (PBNCs) were isolated by Ficoll density centrifugation at 1400 r/min for 30 min and stored at -70 °C before analysis. In order to prevent the contamination, persons with a history of malignant tumors or women in menses or with endometriosis history were excluded from this study.
Total RNA was isolated from MGC-803 cells (5×106) and PBNCs (5×106) with TRIzol reagent (Shanghai Shenergy Biocolor Bioscience and Technology Co., Ltd, SNBC) according to the manufacturer’s protocol. The integrity and concentration of RNA were determined by a gel containing MOPS and formaldehyde.
RT-PCR was based on the methods described by Zheng et al[6], with minor modifications. Briefly, total RNA was heated at 65 °C for 15 min, and then cooled on ice for 10 min. Reverse transcription reaction was performed in a volume of 20 µL containing 1 mmol/L deoxynucleotide triphosphates, 1 mmol/L DTT, 50 mmol/L Tris-HCl (pH 8.3), 60 mmol/L KCl, 3 mmol/L MgCl2, 20 ng Oligo (dT)18 primer (SNBC), 20 units of RNasin (TaKaRa Corp., Dalian, China) and 10 U avian myeloblastosis virus reverse transcriptase (Promega Corp.). The reactions were incubated at 42 °C for 1 h and stopped by heat inactivation at 99 °C for 5 min. cDNA samples were stored at -20 °C until use.
Amplification was performed with 2 µL of cDNA in a total volume of 20 µL containing 1× PCR buffer (10 mmol/L Tris-HCl pH 9.0, 50 mmol/L KCl, 0.1% Triton X-100 and 0.2 mg/mL gelatin), 2 mmol/L MgCl2, 250 µmol/L of each dNTP, 20 pmoL of each primers, and 1 unit Taq DNA polymerase (TaKaRa). DNA from MGC-803 cells was used as a positive control, and negative control contained all components of the RT-PCR reaction except RNA template.
The primer sequences for CD44v6 and CD44s amplification were designed from the published sequences for human CD44 gene[7]. Their location is presented in Figure 1 The sequences for β-actin primer were from Schrewe et al[8].
The sequences of these primers were:
CD44v6 5’-primer: 5’-GACACATATTGCTTCAATGCTTCAGC-3’
CD44v6 3’-primer: 5’-TACTAGGAGTTGCCTGGATGGTAG-3’
CD44s 3’-primer: 5’-GGAATGTGTCTTGGTCTCTGGTAG-3’
β-actin 5’-primer: 5’-CCCTGGACTTCGAGCAAGAGAT-3’
β-actin 3’-primer: 5’-GTTTTCTGCGCAAGTTAGG-3’
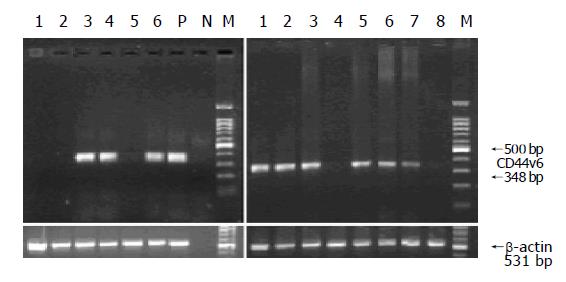
DNA amplification was carried out in the PTC-100TM machine (MJ Research, Watertown, MA). After an initial denaturation step at 94 °C for 3 min, 35 cycles were performed, each at 94 °C for 50 s, at 55 °C for 40 s, and at 72 °C for 1 min, followed by a final extension at 72 °C for 5 min. The expected size of the amplified CD44v6 and CD44s was 348 bp, and the β-actin primers amplified a 531-bp fragment, which was used as an internal control to confirm an equal loading in each slot.
PCR products were analyzed by electrophoresis on 1.8% agarose gel containing 0.5 µg/mL ethidium bromide. DNA fragments were visualized and recorded by a gel scanning system (FR-200, Shanghai FURI Science and Technology Co., Ltd). CD44v6 RT-PCR products from the peripheral blood samples were confirmed in the correct sequences by automated sequencing (Shanghai ShenYou Biotechnology Co., Ltd).
The data were analyzed by χ2 test.
We found that CD44v6 mRNA was detected in 58% (29/50) of healthy volunteers under the appropriate controls. As shown in Figure 2, the amplified fragments in some of the healthy volunteers were similar to those of MGC-803 cells.
CD44v6 expression was found in 17 of 28 samples from males (60.7%) and 12 of 22 samples from females (54.5%). There was no statistical difference in CD44v6 expression in peripheral blood between male and female donors (P>0.5).
In the studies, the average age of the donors was 37.9 years (range 22-69 years, median 35 years). Among them, 24 donors were younger than 35 years and 26 donors were older than 35 years in age. We found that 41.7% (10/24) were CD44v6-positive from the donors (<35 years), while 73.1% (19/26) were CD44v6-positive from the donors (>35 years). There was a statistically significant difference in CD44v6 expression in peripheral blood between the two age groups (P<0.05).
To make sure whether there was any interference between the expression of CD44v6 and CD44s, the expression of CD44s was examined by RT-PCR from all of CD44v6-positive samples. The results showed that CD44s was detected from all of the samples, and the fluorescent intensity of fragments from different samples was similar.
CD44v6 RT-PCR products from the peripheral blood were confirmed to be exactly identical to the published sequences of human CD44 gene by automated sequencing.
CD44 is a widely distributed cell adhesion molecule that is the major cell surface receptor for hyaluronic acid and mediates diverse functions, such as cell-cell and cell-matrix adhesions, lymphocyte homing and T-cell adhesion and activation, embryogenesis, and wound healing. Human CD44 is encoded by a single gene, which consists of 19 exons (Figure 1). CD44s comprises exons 1-5 joined to exons 16-20 to give a molecular weight of 80-90 ku, while CD44v is generated by alternative splicing of exons 6-15 (variant exons 1-10) with higher molecular weights. Alternatively spliced variants expressed in animal tumors have been shown to confer metastatic potential to non-metastatic carcinoma cell lines[9], whereas transfection with CD44v antisense oligonucleotides in the colorectal tumor cell line HT-29 suppressed tumor growth and metastatic behavior in nude mice[10]. Several groups have reported that CD44v6 expression was involved in tumor dissemination and unfavorable prognosis of patients with carcinomas, including cancers of colon, pancreas, breast, prostate, bladder, stomach, etc. The mechanism by which CD44v6 expression in tumor cells is related to invasion and hematogenous metastasis, is not clear. One of the possibilities is that CD44v6 bears the osteopontin binding domain, while malignant cells are often characterized by abundant secretion of osteopontin.
Recently several reports showed that the CD44v6 was expressed not only in malignant tissues but also in many normal tissues and cells. It was reported that elevated levels of CD44v6 expression were noted in peripheral blood lymphocytes of patients with infectious as well as autoimmune diseases[11] and in blood monocytes from healthy donors[12]. In addition, CD44v6 was found in peripheral blood from patients with adenomyosis[13], even on gastric epithelial cells of patients with Helicobacter pylori infection[14].
Based on the studies above, we thought that expression of CD44v6 in peripheral blood from normal people can become as a reliable molecular marker of cancer progression and metastasis. To avoid the contamination of epithelial cells, we discarded the first 10 mL blood samples collected from each donor. The women in menses or with an endometriosis history were excluded from our research to ensure the creditability of the results. Our study showed that CD44v6 expression was found in 29 of 50 peripheral blood samples (58%) from healthy donors using RT-PCR methods. This result seriously doubts about the value of using CD44v6 mRNA to detect tumor cells in peripheral blood. We also detected CD44s mRNA from all CD44v6-positive blood samples and MGC-803 cancer cell line, suggesting that there was no interference between CD44v6 and CD44s expressions.
We found that there was no statistically significant difference in CD44v6 expression between the male and female donors (P>0.5). However, CD44v6 expression was more commonly found in the group aged >35 years than in the group aged <35 years. The precise reason of the different expressions is not clear. One of the probable reasons is that the regulation of body’s gene expression is accurately decreased with age.
In conclusion, the CD44v6 expression in peripheral blood by RT-PCR is not suitable for detection of circulating tumor cells.
We thank staffs of the Department of General Practice, the Affiliated Zhongda Hospital of Southeast University, for their technical assistance in collection of blood samples.
| 1. | Günthert U, Hofmann M, Rudy W, Reber S, Zöller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1237] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 2. | Yamaguchi A, Goi T, Yu J, Hirono Y, Ishida M, Iida A, Kimura T, Takeuchi K, Katayama K, Hirose K. Expression of CD44v6 in advanced gastric cancer and its relationship to hematogenous metastasis and long-term prognosis. J Surg Oncol. 2002;79:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Hefler L, Tempfer C, Haeusler G, Kucera E, Mayerhofer K, Zeillinger R, Reinthaller A, Kainz C. Cytosol concentrations of CD44 isoforms in breast cancer tissue. Int J Cancer. 1998;79:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Stokes GN, Shelton JB, Zahn CM, Kendall BS. Association of CD44 isoform immunohistochemical expression with myometrial and vascular invasion in endometrioid endometrial carcinoma. Gynecol Oncol. 2002;84:58-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Yang L, Xia JG, Zheng SZ, Chen GY. The radioimmunoimaging of 131I labeled CD44v6 monoclonal antibody in nude mice with metastatic focus of gastric cancer in vivo. Acta Nanjing Med Univ. 2002;22:282-283. |
| 6. | Zheng J, Deng YP, Lin C, Fu M, Xiao PG, Wu M. Arsenic trioxide induces apoptosis of HPV16 DNA-immortalized human cervical epithelial cells and selectively inhibits viral gene expression. Int J Cancer. 1999;82:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Goodison S, Yoshida K, Sugino T, Woodman A, Gorham H, Bolodeoku J, Kaufmann M, Tarin D. Rapid analysis of distinctive CD44 RNA splicing preferences that characterize colonic tumors. Cancer Res. 1997;57:3140-3144. [PubMed] |
| 8. | Schrewe H, Thompson J, Bona M, Hefta LJ, Maruya A, Hassauer M, Shively JE, von Kleist S, Zimmermann W. Cloning of the complete gene for carcinoembryonic antigen: analysis of its promoter indicates a region conveying cell type-specific expression. Mol Cell Biol. 1990;10:2738-2748. [PubMed] |
| 9. | Barbour AP, Reeder JA, Walsh MD, Fawcett J, Antalis TM, Gotley DC. Expression of the CD44v2-10 isoform confers a metastatic phenotype: importance of the heparan sulfate attachment site CD44v3. Cancer Res. 2003;63:887-892. [PubMed] |
| 10. | Reeder JA, Gotley DC, Walsh MD, Fawcett J, Antalis TM. Expression of antisense CD44 variant 6 inhibits colorectal tumor metastasis and tumor growth in a wound environment. Cancer Res. 1998;58:3719-3726. [PubMed] |
| 11. | Wittig B, Seiter S, Schmidt DS, Zuber M, Neurath M, Zöller M. CD44 variant isoforms on blood leukocytes in chronic inflammatory bowel disease and other systemic autoimmune diseases. Lab Invest. 1999;79:747-759. [PubMed] |
| 12. | Braumüller H, Gansauge S, Ramadani M, Gansauge F. CD44v6 cell surface expression is a common feature of macrophages and macrophage-like cells - implication for a natural macrophage extravasation mechanism mimicked by tumor cells. FEBS Lett. 2000;476:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Lin Z, Cho S, Jeong H, Kim H, Kim I. Immunohistochemical analysis of CD44s and CD44v6 in endometriosis and adenomyosis: comparison with normal, hyperplastic, and malignant endometrium. J Korean Med Sci. 2001;16:317-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Fan X, Long A, Goggins M, Fan X, Keeling PW, Kelleher D. Expression of CD44 and its variants on gastric epithelial cells of patients with Helicobacter pylori colonisation. Gut. 1996;38:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |