Published online Jun 7, 2005. doi: 10.3748/wjg.v11.i21.3281
Revised: March 10, 2004
Accepted: April 13, 2004
Published online: June 7, 2005
AIM: To investigate the tissue distribution, urinary and fecal excretions of 125I-lidamycin (125I-C-1027) in mice and its biliary excretion in rats.
METHODS: The total radioactivity assay (RA method) and the radioactivity assay after precipitation with 200 mL/L trichloroacetic acid (TCA-RA method) were used to dete-rmine the tissue distribution, and the urinary and fecal excretions of 125I-C-1027 in mice and its biliary excretion in rats.
RESULTS: Tissue concentrations reached the peak at the fifth minute after administration of 125I-C-1027 to mice. The highest concentration was in kidney, and the lowest in brain at all test-time points. The organs of the concentr-ations of 125I-C-1027 from high to low were kidney, lung, liver, stomach, spleen, uterus, ovary, intestine, muscle, heart, testis, fat, and brain in mice. The accumulative excretion amounts of 0-24 h, and 0-96 h after administration of 125I-C-1027 were 68.36 and 71.64% in urine, and 2.60 and 3.21% in feces of mice, respectively, and the accumulative excretion amount of 0-24 h was 3.57% in bile in rats.
CONCLUSION: Our results reflect the characteristics of the tissue distribution, urinary and fecal excretions of 125I-C-1027 in mice and the biliary excretion of 125I-C-1027 and its metabolites in rats, and indicate that 125I-C-1027 and its metabolites are mainly distributed in kidney, and excreted in urine.
- Citation: Liu YP, Li QS, Huang YR, Liu CX. Tissue distribution and excretion of 125I-lidamycin in mice and rats. World J Gastroenterol 2005; 11(21): 3281-3284
- URL: https://www.wjgnet.com/1007-9327/full/v11/i21/3281.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i21.3281
Lidamycin (C-1027) is a new kind of macromolecular antitumor antibiotics, produced by Streptomyces globisporus in soil, consisting of a noncovalently bound apoprotein and a labile chromophore which is responsible for most of the biological activities[1-5]. C-1027 could exert remarkable inhibition on the growth of human liver and colon cancers[6,7], and shows a highly potent cytotoxicity to cultured cancer cells and a marked DNA cleaving ability[8]. The protein moiety of C-1027 has a single polypeptide chain cross-linked by two disulfide bonds with a molecular weight of 10500 Da[9,10]. Like other enediyne agents, antibiotic C-1027 is believed to exert its biological activity through the induction of cellular DNA damage[11-13]. Preclinical studies on the pharmacodynamics, pharmacokinetics and toxicology have exhibited that C-1027 appears to be a very promising anticancer candidate, and is entering clinical trial in China. The study on the pharmacokinetics of lidamycin has been finished, and would be reported. In this paper, we investigated the distribution and excretion characteristics of 125I-lidamycin in different organs of mice and rats.
C-1027 (Lot: 20020525), with a purity of 95.0%, was produced by the Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical University (Beijing, China). 125I-C-1027, which was radio-iodinated by the Iodogen method[14], had a specific radioactivity of 7.45 mCi (275.65 MBq)/mg. The radiochemical purity was more than 95.0%. Iodogen was from the Academy of Military Medical Sciences (Beijing, China). Trichloroacetic acid (analytical grade) was provided by the Chemical Company (Beijing, China), and sodium chloride solution (9 g/L) was purchased from Dazhong Pharmaceutical Company (Tianjin, China). Gamma counter (FJ630G/12 model) was produced by the Beijing Nuclear Company (Beijing, China). This chromat-ographic system (LC-6A, Shimadzu, Japan) consists of a pump (LC-6AT), a temperature box and a variable wavelength UV detector (Spectra 100, Shimadzu, Japan). Sephadex G-50 column (300 mm×7.8 mm2 I.D.) was purchased from Pharmacia Company (USA). Distilled water, prepared from demineralized water, was used throughout the study.
Kunming mice (body weight, 17-24 g) and Wistar rats (body weight, 190-250 g), males and females, were purchased from the Center of Experimental Animals of Tianjin Institute of Pharmaceutical Research (Certificate No. 20020804, Tianjin, China). They were raised at least 1 wk before the experiment.
Iodogen (100 μg) in 100 μL of chloroform was placed in a sample tube and evaporated to dryness with nitrogen gas. Fifty micrograms of C-1027 and 50 µL of Na125I (74 MBq) were pipetted, mixed, and allowed to react at 15 °C for 30 min[14]. The mixture was chromatographed on Sephadex G-50 column. The mobile phase consisted of 0.05 mol/L sodium dodecyl sulfate phosphate buffer solution (pH 7.0) at a flow rate of 0.8 mL/min. The eluted fractions, detected by a gamma counter as the same chromatographic behavior as standard C-1027 and Na125I, were the components of 125I-C-1027 and Na125I, respectively. The fraction, collected from 10 to 12.5 min, was a single radioactive peak of 125I-C-1027, and was concentrated to a specific activity and applied for the study.
Serum sample solution (100 µL), which was prepared by diluting the weighed tissue five times with 0.9% sodium chloride solution, was directly determined by a gamma counter for radioactivity assay (RA method). To 100 µL of serum sample, 100 µL of 20% trichloroacetic acid was added. The mixed solution was homogenized, precipitated, and centrifuged at 1500 r/min for 10 min. The supernatant was removed, and the precipitated fraction was detected by a gamma counter.
The mice were randomly divided into three groups. Each group contained six mice. The mice were killed at 5, 30 min and 2, and 6 h after tail vein injection of 125I-C-1027 at the dose of 50 µg/kg, respectively. Each of the tissues from heart, liver, spleen, lung, kidney, brain, muscle, intestines, fat, ovary, testis, and uterus was isolated and prepared for the assay.
125I-C-1027 (50 µg/kg) was administered to mice by intra-venous injection. The animals were raised in metabolic cages. The urinary and fecal samples were collected at the fixed time intervals from 0 to 1, 1 to 2, 2 to 4, 4 to 6, 6 to 8, 8 to 12, 12 to 24, 24 to 36, 36 to 48, 48 to 72, and 72 to 96 h, respectively. The total volume of urine was measured. The feces was freeze-dried, weighed, and pulverized with a mortar or pestle. All samples were stored at -20 °C until analysis.
The rats, which were anesthetized by i.p. injection of urethan (1.2 g/kg), were operated by the bile duct-cannulation method. Then, the animals were administered 125I-C-1027 (50 µg/kg) by tail vein injection. The bile samples were collected at the fixed time intervals from 0 to 1, 1 to 2, 2 to 4, 4 to 6, 6 to 8, 8 to 12, and 12 to 24 h. The total volume was measured, and the samples were kept at -20 °C until analysis.
The t-test was used to analyze the tissue distribution data. The data were expressed as mean±SD.
Stock solution (2.0 mg/L) of 125I-C-1027 (275.65 MBq/L) and C-1027 was prepared in water, and stored at -20 °C. The stock solution of 125I-C-1027 (2.0 mg/L), containing 275.65 MBq/L of 125I-C-1027, was prepared into the serial concentrations of standard solution of 0.5, 2.5, 5.0, 50.0, 200.0, 500.0, and 1000.0 μg/L with 0.9% sodium chloride solution. The standard solution was used to prepare standard curves. The serial concentrations of calibration curves were prepared with blank tissues of mice instead of 0.9% sodium chloride solution as mentioned above. Quality control (QC) samples were prepared into concentrations of 5.0, 50.0, and 200.0 μg/L in tissue. The regression equations, the concentrations (x) vs response values (y), were as follows:
y= 90.2+1 103.9x (n = 8, r = 0.9997) for the standard 125I-C-1 027, where r represents correlation coefficient.
y = 102.4+1 058.1x (n = 8, r = 0.9995) for serum at the concentration range from 0.5 to 100.0 μg/L, and
Heart: y = 67.6+1 235.8x (n = 7, r = 0.9996),
Liver: y = 9.60+1 219.9x (n = 8, r = 0.9999),
Kidney: y = 48.9+1 237.7x (n = 7, r = 0.9999),
Brain: y = 75.4+1 220.9x (n = 7, r = 0.9998),
Muscle: y = 39.2+1 225.9x (n = 6, r = 0.9999),
Urine: y = 76.0+1 232.4x (n = 7, r = 0.9999),
Feces: y = 44.6+1 232.8x (n = 6, r = 0.9999),
Bile: y = 57.9+1 278.9x (n = 5, r = 0.9998) for each tissue at concentration range from 0.5 to 100.0 μg/L.
The precision and accuracy of the assay were evaluated by assaying QC samples (5.0, 50.0, and 200.0 μg/L) in six replicates on three different days.
The precision, which was evaluated by a one-way analysis of variance (ANOVA), was defined as the relative standard deviation (RSD). The intra-day and inter-day mean square of variance was used to calculate RSD values. The RSD was less than 5.0% for intra-day and less than 10.0% for inter-day assay at the range from 0.5 to 1000.0 μg/L for each tissue, urine, feces and bile sample. The accuracy was expressed as the recovery (more than 98%) (Table 1). The validation results satisfied the need of bioanalysis[15,16].
| Nominal concentration (mg/L) | Measured concentration (mg/L) | Recovery (%) | Precision (%) | |
| Intra-d | Inter-d | |||
| 5.0 | 4.91±0.04 | 98.2 | 4.12 | 9.78 |
| 50.0 | 49.64±0.07 | 99.3 | 4.05 | 8.01 |
| 200.0 | 199.3±0.4 | 99.6 | 3.18 | 7.83 |
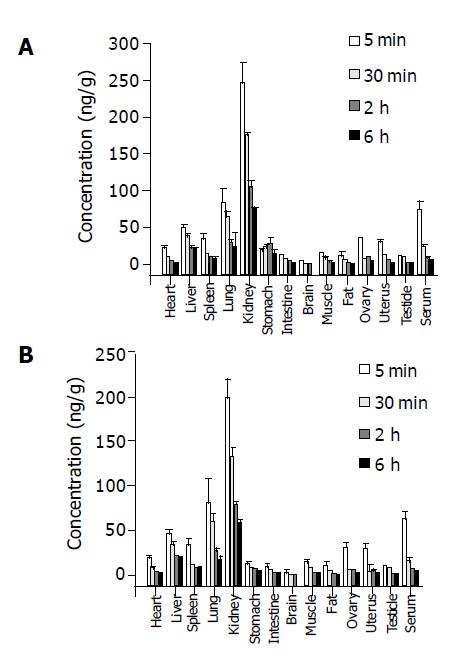
125I-C-1027 was observed in different tissues of mice after intravenous administration of 125I-C-1027 (50 µg/kg). Figures 1A and 1B represent the graphs of the tissue distribution of radioactivity of 125I-C-1027 by RA and TCA-RA methods after intravenous injection to mice, respectively. The conce-ntrations of 125I-C-1027 were the highest in kidney. The unchanged drug could not be detected except in kidney after 36 h. The results, obtained by RA and TCA-RA methods, were analyzed by t-test. There was a significant difference between RA and TCA-RA methods (P<0.05). The results measured by RA method were higher than those by TCA-RA method. The RA method determined the total radioactivity, and the TCA-RA method detected the precipitated fractions and could remove the interference of decomposed compounds with a small molecular weight. The TCA-RA method was a better method to assay 125I-C-1027 in tissues.
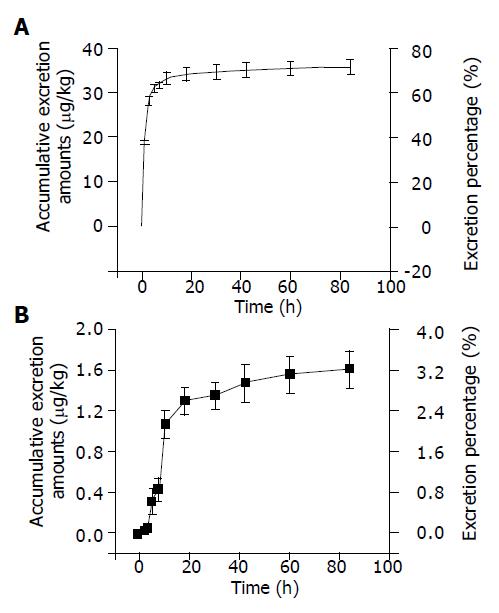
The excretion-time curves of urine and feces, assayed by RA method after intravenous injection of 125I-C-1027 (50 µg/kg) to mice, are plotted in Figure 2. The accumulative excretion percentages were 71.64 and 3.21% from urine and feces within 96 h, respectively. The urine excretion was the major pathway of radioactive materials.
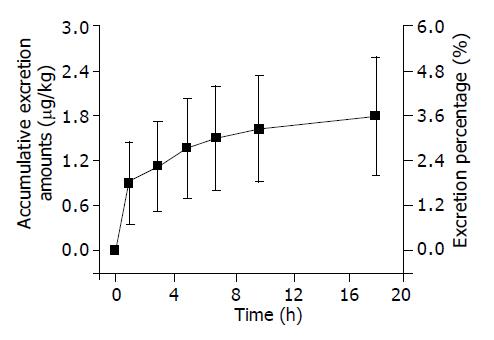
The results determined after iv. injection of 125I-C-1027 (50 µg/kg) to rats by RA method are displayed in Figure 3. The accumulative excretion amount was 3.57% in bile within 24 h. It indicated that the bile excretion was a subsidiary pathway of radioactive materials.
The tissue distribution experiment showed that 125I-C-1027 was widely distributed in most tissues of mice after intravenous administration of it at the dose of 50 µg/kg. The drug concentration was the highest in kidney. The concentrations of 125I-C-1027 in fat, brain, ovary, testis, and uterus tissues were lower than those in other tissues. The level of 125I-C-1027 could not be detected except in kidney after 36 h of administration. The concentrations in most tissues reached the peak values at the fifth min after administration. The accumulative excretion percentages were 71.64, 3.21, and 3.75% in urine, feces, and bile, respectively. The kidney had a high concentration of 125I-C-1027 in our experiment, multi-dosage tissue distribution test was done within 72 h, and we found that there was a little accumulation in kidney.
RA method determined the total radioactivity, and could not distinguish 125I-C-1027 from its decomposed products. TCA-RA method could remove 125I and its decomposed products with a small molecular weight, and has become an accepted method[17]. TCA-RA method could further reflect the tissue distribution characteristics of C-1027 compared with RA method. So, TCA-RA method is better than RA method.
| 1. | Hu JL, Xue YC, Xie MY, Zhang R, Otani T, Minami Y, Yamada Y, Marunaka T. A new macromolecular antitumor antibiotic, C-1027. I. Discovery, taxonomy of producing organism, fermentation and biological activity. J Antibiot (Tokyo). 1988;41:1575-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Otani T, Minami Y, Marunaka T, Zhang R, Xie MY. A new macromolecular antitumor antibiotic, C-1027. II. Isolation and physico-chemical properties. J Antibiot (Tokyo). 1988;41:1580-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 56] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Zhen YS, Ming XY, Yu B, Otani T, Saito H, Yamada Y. A new macromolecular antitumor antibiotic, C-1027. III. Antitumor activity. J Antibiot (Tokyo). 1989;42:1294-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 84] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Matsumoto T, Okuno Y, Sugiura Y. Specific interaction between a novel enediyne chromophore and apoprotein in macromolecular antitumor antibiotic C-1027. Biochem Biophys Res Commun. 1993;195:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Otani T. Conformation studies on and assessment by spectral analysis of the protein-chromophore interaction of the macromolecular antitumor antibiotic C-1027. J Antibiot (Tokyo). 1993;46:791-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | He QY, Jiang B, Li DD, Zhen YS. Effects of lidamycin on apoptotic gene expressions and cytoskeleton in human hepatoma bel-7402 cells. AiZheng. 2002;21:351-355. [PubMed] |
| 7. | Cui DP, Wang Z, Li DD. Effect of lidamycin on the expression of genes involved in invasion regulation in HCT-8 human colon cancer cells. YaoXue XueBao. 2001;36:246-249. [PubMed] |
| 8. | Liu JS, Kuo SR, Yin X, Beerman TA, Melendy T. DNA damage by the enediyne C-1027 results in the inhibition of DNA replication by loss of replication protein A function and activation of DNA-dependent protein kinase. Biochemistry. 2001;40:14661-14668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Shao RG, Zhen YS. Relationship between the molecular composition of C1027, a new macromolecular antibiotic with enediyne chromophore, and its antitumor activity. YaoXue XueBao. 1995;30:336-342. [PubMed] |
| 10. | Zhou CS, Xu LN, Jiang M, Zhen YS. A monoclonal antibody directed against an enediyne antitumor antibiotic and its preliminary application. YaoXue XueBao. 1997;32:28-32. [PubMed] |
| 11. | Xu YJ, Li DD, Zhen YS. Mode of action of C-1027, a new macromolecular antitumor antibiotic with highly potent cytotoxicity, on human hepatoma BEL-7402 cells. Cancer Chemother Pharmacol. 1990;27:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Wang Z, He Q, Liang Y, Wang D, Li YY, Li D. Non-caspase-mediated apoptosis contributes to the potent cytotoxicity of the enediyne antibiotic lidamycin toward human tumor cells. Biochem Pharmacol. 2003;65:1767-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Totsuka R, Aizawa Y, Uesugi M, Okuno Y, Matsumoto T, Sugiura Y. RNA cleavage by C-1027 chromophore, an enediyne antitumor antibiotic: high selectivity to an anticodon arm. Biochem Biophys Res Commun. 1995;208:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Tang ZM, Liu XW, Xu LP, Shan CW, Song QS. Pharmacokinetics and tissue distribution of human recombinant interleukin-2 in mice. Zhongguo Yao Li Xue Bao. 1994;15:51-56. [PubMed] |
| 15. | Shah VP, Midha KK, Dighe S, McGilveray IJ, Skelly JP, Yacobi A, Layloff T, Viswanathan CT, Cook CE, McDowall RD. Analytical methods validation: bioavailability, bioequivalence and pharmacokinetic studies. Conference report. Eur J Drug Metab Pharmacokinet. 1991;16:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 382] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 16. | Liu CX, Wei GL, Li QS. Methodology study of validation for bioanalysis in studies on pharmacokinetics and bioavailability. Asian J Drug Metab Pharcokinet. 2001;1:279-286. |