INTRODUCTION
Ornithine decarboxylase (ODC) catalyzes the initial step in the biosynthesis of polyamines and ODC activities are associated with malignant status[1]. Overexpression of ODC could lead to cell transformation, tumor invasion, and metastasis[2-4]. A number of studies demonstrated increased expression of ODC in a variety of cancers including colorectal cancer[1].
Invasion and metastasis are a multistep process[5]. Degradation of basement membranes and extracellular matrix is crucial for invasion and metastasis of cancer cells. Basement membranes contain type IV collagen, laminin, heparan sulfate proteoglycan, fibronectin, and other components[5]. Degradation of type IV collagen, a major component of basement membrane, is thought to be a prerequisite for cancer cell invasion[6]. Increased expression or activity of matrix metalloproteinases (MMPs) has been linked to malignancy and tumor cell invasion[7-9]. At least 24 MMPs have been reported so far[7-9]. MMP-2 (72 ku type IV collagenase) which preferentially degrades type IV collagen has been shown to play an important role in invasion and metastasis[5,8]. Several studies have demonstrated increased expression of MMP-2 related to invasion and metastasis of colorectal cancer[10-13].
Mitogen-activated protein (MAP) kinase is a key enzyme of the signal transduction pathways triggered by extracellular signals, including mitogens, growth factors, and cytokines[14-16]. Erk1/2, extracellular signal-regulated kinases that are also termed as p44 and p42 MAP kinases, are ubiquitously expressed. p38 MAP kinase is activated by a variety of cellular stresses, including osmotic shock, inflammatory cytokines, lipopolysaccharides, UV irradiation, and growth factors[17]. Limited information is available concerning MAP kinase expression in human surgically removed colon cancer tissues and its adjacent normal tissues[18]. Hoshino et al[18] showed constitutive activation of MAP kinases in 138 human cancer cell lines, and primary tumor tissues including colon cancer. However, it was reported that the activities of Erk1/2, and p38 MAP kinase were downregulated in the majority of human colon cancers[19]. Sakakura et al[20] also reported infrequent activation of MAP kinase in human colon cancer. Recently, the p38 MAP kinase signaling pathway was shown to be important for the induction of MMP-1 and MMP-9 by extracellular stimuli[21,22]. Therefore, it is imperative to analyze the expression levels of MAP kinases (Erk1/2 and p38) in human surgically removed colon cancer tissues and its adjacent normal tissues.
Colon cancer is one of the most common malignancies in the world and is incurable in its advanced stages[23]. Therefore, it is important to understand the mechanism of invasion and metastasis, and to develop better treatments to prevent or cure metastatic colon cancer. As colon cancer has increased expression levels of ODC, MMP-2, and MAP kinase, these three molecules are the candidate targets for treatment of colon cancer. It is important to clarify a correlation among expressions of these three molecules. We previously found that ODC overexpression in fibroblasts led to cellular transformation and invasion with concomitant induction of MMP-2 and Erk1/2[3]. Little information is available concerning the correlation among ODC, MMP-2, and MAP kinase expressions in cancer in vitro and in vivo. The aim of this study was to investigate the expressions of ODC, MAP kinase, and MMP-2, and further to elucidate the relationship among these expressions in human colon cancer. We discussed the strategies of colon cancer treatment or prevention using α-difluoromethylornithine (DFMO), an irreversible inhibitor of ODC.
MATERIALS AND METHODS
Reagents
Antibodies against phospho-Erk and phospho-p38 MAP kinase were obtained from New England Biolabs (Beverly, MA, USA). MMP-2 ELISA system and DL-[1-14C] ornithine were obtained from Amersham Pharmacia Biotech (Buckinghamshire, UK).
Samples
Paired specimens (tumor and non-cancer tissues adjacent to tumor) were obtained from a total of 58 colon cancer patients who had undergone surgical operation for adenocarcinoma at Saitama Medical University Medical Center. Written informed consent was obtained from all patients before surgical operation. All specimens were immediately stored at -80 °C until use.
ODC enzyme assay
ODC activity was determined using [1-14C]-ornithine as a substrate as described previously[3].
MMP-2 ELISA and Western blot analysis
Tissue lysates were prepared by sonicating tissues in lysis buffer (10 mmol/L Tris-HCl buffer, pH 7.5, containing 1 mmol/L EDTA, 0.5 μg/mL aprotinin, 1 μg/mL leupeptin, and 0.2 mmol/L PMSF) and centrifuged at 12000 g for 10 min at 4 °C. Measurement of protein concentration in supernatant was performed using a Bio-Rad protein assay kit (Hercules, CA, USA). Western blotting was performed as previously described[24]. MMP-2 ELISA was performed according to the manufacturer’s protocol. Quantification of the bands was performed using a NIH image.
Statistical analysis
The results were expressed as mean±SD from three independent experiments. The significance was determined by the paired t-test or Pearson’s test. Statistical analyses were performed using the Statview 4.5 software (Abacus Concepts, Inc., Berkeley, CA, USA).
RESULTS
ODC activity in colon cancer tissues
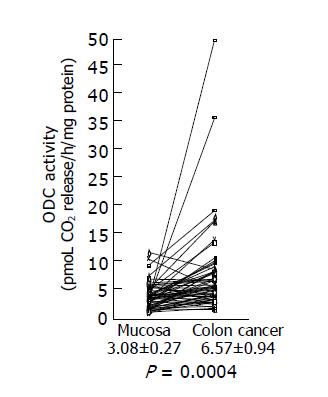
We examined ODC activities in surgically excised human colon cancer (Figure 1). A remarkable increase in ODC activities was seen in colon cancers. Using the paired t-test, a significant increase in ODC activities in colon cancer tissues (6.57±0.94 pmoL CO2 release/h/mg protein) was shown (P = 0.0004 by the paired t-test, n = 58), compared with those observed in adjacent non-cancer tissues (3.08±0.27 pmoL CO2 release/h/mg protein). Increased ODC activity over the normal mean±SD level of colon cancer was seen in 17 of 58 (29.3%) cases.
Figure 1 ODC activity in colon cancer tissues and adjacent normal tissues.
ODC activities in colon cancer tissues and adjacent normal tissues were assayed as described in Materials and methods, and the data are shown as mean±SD (pmol CO2 release/h/mg protein).
MMP-2 levels in colon cancer tissues
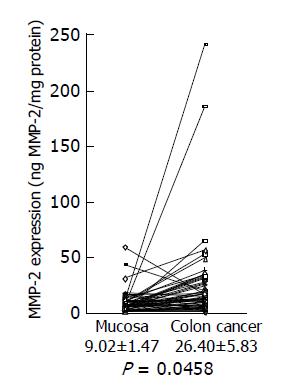
Next, we investigated MMP-2 expression in colon cancer using a MMP-2 ELISA kit. A significant increase in MMP-2 levels was shown in colon cancer tissues (26.40±5.83 ng/mg protein), compared with adjacent non-cancer tissues (9.02±1.47 ng/mg protein) (Figure 2) (P = 0.046 by the paired t-test, n = 58). Increased MMP-2 expression over the level of the normal mean±SD in colon cancer was observed in 28 of 58 (48.3%) cancers.
Figure 2 MMP-2 expression levels in colon cancer tissues and adjacent normal tissues.
MMP-2 expression levels in colon cancer tissues and adjacent normal tissues were analyzed by MMP-2 ELISA, and the data are shown as mean±SD (ng MMP-2/mg protein).
Expressions of phosphorylated Erk and p38 MAP kinase in colon cancer tissues
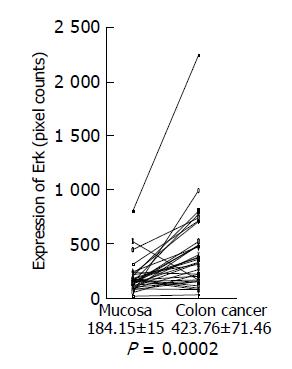
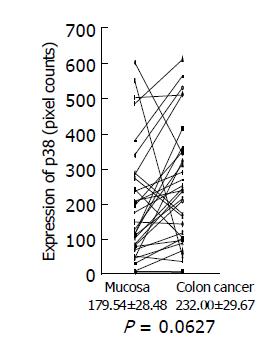
Expression of phosphorylated Erk1/2 and p38 MAP kinase was analyzed by Western blotting using the antibodies against phosphorylated Erk and p38 MAP kinase, respectively. Because of limited amount of tumor tissues, 33 out of 58 samples were used for this experiment. Using the paired t-test, a significant increase in phosphorylated Erk1/2 was shown in colon cancer (423.76±71.46 pixel counts) (P = 0.0002, n = 33), compared with the adjacent tissues (184.15±15.0 pixel counts) (Figure 3). Mean value of phosphorylated p38 MAP kinase had a tendency to increase in colon cancer (232.00±29.67 pixel counts in colon cancer tissues vs 179.54±28.48 pixel counts in adjacent tissues), although P value did not reach a significant level (P = 0.0627) (Figure 4).
Figure 3 Erk expression in colon cancer tissues and adjacent normal tissues.
Expression of phosphorylated Erk1/2 in colon cancer tissues and adjacent normal tissues was analyzed by Western blotting using a specific antibody against phosphorylated Erk1/2, quantitated using a NIH image, and shown as mean±SD (pixel counts).
Figure 4 p38 MAP kinase expression in colon cancer tissues and adjacent normal tissues.
Expression of phosphorylated p38 MAP kinase in colon cancer tissues and adjacent normal tissues was analyzed by Western blotting using a specific antibody against phosphorylated p38 MAP kinase, quantitated using a NIH image, and are shown as mean±SD (pixel counts).
Correlation between ODC activity, MMP-2 and Erk expressions
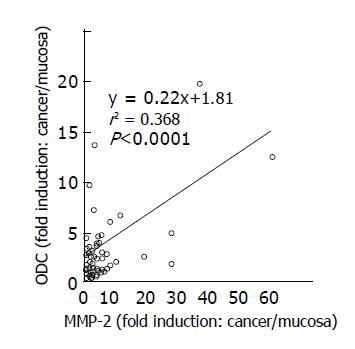
A significant correlation was noted between ODC activity and MMP-2 expression in colon cancer tissues and adjacent normal tissues (r2 = 0.368, P<0.001, n = 58) (Figure 5). Although a correlation between ODC activity and phosphorylated Erk1/2 expression was not found (r2 = 0.028, P = 0.35) in colon cancers (Figure 6), increased expression of phosphorylated Erk1/2 over the level of the normal mean±SD level was observed in 9 of 33 (27.3%) colon cancers (Figure 3). Increased expression of phosphorylated p38 MAP kinase over the level of the normal mean±SD was observed only in 3 of 33 (9.1%) colon cancers (Figure 4).
Figure 5 Relationship between ODC activities and MMP-2 expression in colon cancer tissues.
Correlation between ODC activities and MMP-2 expression levels in cancer tissues, compared to those in adjacent normal tissues was shown. Statistical significance was analyzed as described in Materials and methods.
Figure 6 Relationship between ODC activities and Erk expression in colon cancer tissues.
Correlation between ODC activities and Erk1/2 expression levels in cancer tissues, compared to those in adjacent normal tissues was shown. Statistical significance was analyzed as described in Materials and methods.
DISCUSSION
In the present study we showed that ODC activities, MMP-2 levels, and Erk1/2 expression had 2.13-, 2.93-, and 2.30-fold increase in colon cancer tissues, respectively, compared to its adjacent normal tissues. Increased ODC activity and MMP-2 levels over the normal mean±SD level in colon cancer were observed in 17 (29.3%) and 28 (48.3%) out of 58 colon cancer patients, respectively. Our results were essentially consistent with the previous report concerning increased ODC activities[1] and increased expression of MMP-2 in colon cancer[10-13]. The data concerning Erk1/2 expression were consistent with the results of a previous report[18], but not with other reports[19,20]. The reason for the inconsistency is not clear. A new finding in the present study was that there was a relationship between expressions of ODC and MMP-2 in colon cancer. We previously demonstrated that ODC overexpression induced MMP-2 and Erk1/2 expressions in fibroblasts[3]. So, it is reasonably assumed that ODC expression induces MMP-2 expression in human colon cancer. A linkage of ODC and MMP-2 expressions very well explains a basis of invasive phenotype of cancers which overexpress ODC.
Metastasis is a multistep process, which includes local invasion, intravasation and extravasation of cancer cells[5]. Major functional contribution of MMPs is facilitation of degradation of basement membranes between primary tumor and distant metastasis sites. As MMP-2 preferentially degrades type IV collagen, a major component of basement membrane, MMP-2 has been thought to play a major role in invasion process[5,8]. Recent evidence indicates that MMPs including MMP-2 play a much broader role in metastasis both before and after the degradation of basement membrane[25]. For example, MMP-2 contributes to the initiation of tumor growth at both primary and metastasis sites. This may involve regulation of growth environment by regulating access to growth factors from the extracellular matrix surrounding the tumor, either directly or via a proteolytic cascade. The role of MMPs in angiogenesis is considered to be very important, as angiogenesis is required for tumor growth and invasion. Recent studies indicated that MMPs, especially MMP-2, played a pivotal role in angiogenesis[26-28]. In fact, mice deficient in MMP-2 exhibited reduced angiogenesis in vivo[29]. As MMP-2 overexpression in colon cancer has been found to be crucial for cancer invasion and metastasis[30], suppression of ODC using DFMO or DFMO in combination with other chemopreventives may be a rational approach to the treatment of colon cancer.
MAP kinase pathways play a major role in converting mitogenic and stress stimuli into nuclear responses. Constitutive activation of the 41-/43-ku MAP kinase (Erk1/2) signaling pathway was shown in human tumors[18]. In the present study we also found increased expression of phosphorylated Erk1/2 in colon cancer. As ODC and Erk1/2 expressions were not correlated in the present study, the data suggested that Erk1/2 expression was not a downstream signal transduction event after ODC expression in human colon cancer. Wang et al[19] reported that the activities of Erk1/2, JNK1 and p38 MAP kinase were downregulated in the majority of human colon cancers. The reason for the discrepancy of our result and their result concerning Erk1/2 is not clear. They discussed that protein kinases other than MAP kinases might play a more crucial role in colon carcinogenesis[19]. In the present study we showed that increased expression of phosphorylated p38 MAP kinase over the level of the normal mean±SD was observed only in 3 of 33 (9.1%) colon cancers. This result might be inconsistent with that of their report[19]. p38 MAP kinase was reported to be involved in other MMP (MMP-1 and MMP-9) expression[21,22]. However, our result and Wang’s result[19], taken together, suggest that p38 MAP kinase may not be involved in carcinogenesis or invasive phenotype or MMP-2 expression in human colon cancer.
As colon cancer is still incurable at advanced stages, new strategies for prevention and treatment are desired[23]. In the 1980s, DFMO was abandoned due to limited efficacy and significant adverse effects such as nausea, vomiting, abdominal pain, diarrhea, and hearing loss in phase II clinical trials[23,31,32]. In the following decade, DFMO was resuscitated as a chemopreventive agent[33,34]. DFMO (0.2-1.0 g/m2/d) effectively inhibited ODC activities in target organs such as the colorectum in patients with a personal or family history of colorectal cancer without side effects such as cytotoxicity[35,36]. DFMO is now being tested in phase II/III trials involving patients at risk for colorectal cancer[23]. Recent studies have shown that DFMO had synergistic reductions in intestinal neoplasia when DFMO was administered with cyclooxygenase inhibitors such as piroxicam or aspirin even as the doses of both agents were reduced as much as 50%[37-41]. Thus, DFMO in combination with other chemopreventive agents should be evaluated for the efficacy against colon cancer. As PD 184352 (MEK inhibitor)[42] or BB-94 (MMP inhibitor)[43] has been available for clinical trial, DFMO in combination of PD 184352 or BB-94 may be a rational approach to the treatment of colon cancers overexpressing ODC.