Published online Jan 14, 2005. doi: 10.3748/wjg.v11.i2.280
Revised: January 12, 2004
Accepted: March 12, 2004
Published online: January 14, 2005
AIM: To investigate the apoptosis of implanted primary gastric cancer cells in nude mice induced by resveratrol and the relation between this apoptosis and expression of bcl-2 and bax.
METHODS: A transplanted tumor model was established by injecting human primary gastric cancer cells into subcutaneous tissue of nude mice. Resveratrol (500 mg/kg, 1000 mg/kg and 1500 mg/kg) was directly injected beside tumor body 6 times at an interval of 2 d. Then changes of tumor volume were measured continuously and tumor inhibition rate of each group was calculated. We observed the morphologic alterations by electron microscope, measured the apoptotic rate by TUNEL staining method, detected the expression of apoptosis-regulated genes bcl-2 and bax by immunohistoch-emical staining and PT-PCR.
RESULTS: Resveratrol could significantly inhibit carcinoma growth when it was injected near the carcinoma. An inhibitory effect was observed in all therapeutic groups and the inhibition rate of resveratrol at the dose of 500 mg/kg, 1000 mg/kg and 1500 mg/kg was 10.58%, 29.68% and 39.14%, respectively. Resveratrol induced implanted tumor cells to undergo apoptosis with apoptotic characteristics, including morphological changes of chromatin condensation, chromatin crescent formation, nucleus fragmentation. The inhibition rate of 0.2 mL of normal saline solution, 1 500 mg/kg DMSO, 500 mg/kg resveratrol, 1000 mg/kg resveratrol, and 1500 mg/kg resveratrol was 13.68±0.37%, 13.8±0.43%, 48.7±1.07%, 56.44±1.39% and 67±0.96%, respectively. The positive rate of bcl-2 protein of each group was 29.48±0.51%, 27.56±1.40%, 11.86±0.97%, 5.7±0.84% and 3.92±0.85%, respectively by immunohistochemical staining. The positive rate of bax protein of each group was 19.34±0.35%, 20.88±0.91%, 40.02±1.20%, 45.72±0.88% and 52.3±1.54%, respectively by immunohistochemical staining. The density of bcl-2 mRNA in 0.2 mL normal saline solution, 1500 mg/kg DMSO, 500 mg/kg resveratrol, 1000 mg/kg resveratrol, and 1500 mg/kg resveratrol decreased progressively and the density of bax mRNA in 0.2 mL normal saline solution, 1500 mg/kg DMSO, 500 mg/kg resveratrol, 1000 mg/kg resveratrol, and 1500 mg/kg increased progressively with elongation of time by RT-PCR.
CONCLUSION: Resveratrol is able to induce apoptosis of transplanted tumor cells. This apoptosis may be mediated by down-regulating apoptosis-regulated gene bcl-2 and up-regulating the expression of apoptosis-regulated gene bax.
- Citation: Zhou HB, Chen JJ, Wang WX, Cai JT, Du Q. Anticancer activity of resveratrol on implanted human primary gastric carcinoma cells in nude mice. World J Gastroenterol 2005; 11(2): 280-284
- URL: https://www.wjgnet.com/1007-9327/full/v11/i2/280.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i2.280
Bcl-2 family plays a crucial role in the control of apoptosis. The family includes a number of proteins which have homologous amino acid sequences, including anti-apoptotic members such as bcl-2 and bcl-xl, as well as pro-apoptotic members including bax and bad. In in vitro experiments, overexpression of bcl-2 has been shown to inhibit apoptosis, but overexpression of bax has been shown to promote apoptosis.
Resveratrol, a phytoalexin found in grapes, fruits, and root extracts of the weed Polygonum cuspidatum, is an important constituent of Chinese folk medicine. Indirect evidence suggests that the presence of resveratrol in white and rose wine may be helpful to reduce risks of coronary heart disease which would be achieved by a moderate wine consumption. This effect has been attributed to the inhibition of platelet aggregation and coagulation, in addition to the anti-oxidant and anti-inflammatory activity of resveratrol. Moreover, a recent report showed that resveratrol was a potent cancer chemopreventive agent in three major stages of carcinogenesis. We found resveratrol was able to induce apoptosis in primary gastric cancer in vitro. This apoptosis may be mediated by down-regulating the expression of apoptosis-regulated gene bcl-2 and up-regulating the expression of apoptosis-regulated gene bax.
This study was to investigate the apoptosis of implanted tumor of primary gastric cancer cells in nude mice induced by resveratrol and the relation between this apoptosis and expression of bcl-2 and bax in vivo and to provide the theoretical and methodological basis for its clinical application.
Resveratrol was obtained from Sigma Chemical Co. Ltd and dissolved in DMSO. In situ cell detection kit, anti-bcl-2 and anti-bax monoclonal antibodies were purchased from Beijing Zhongshan Biotechnology Co. Ltd. Balb/C female nude mice (4 wk old, 16-18 g) were obtained from Chinese Academy of Medical Sciences.
Cell culture Fresh samples from a patient with low-differentiation gastric cancer were obtained at operating. A single-cell suspension of tumor cells with the concentration of 5×105/mL was prepared for seeding. Primary gastric cancer cells were artificially purified after cultured with pancreatic proteinase.
Tumor implanted into nude mice A transplanted tumor model was established by injecting 1×109/L human primary gastric cancer cells into subcutaneous tissues of nude mice. After 10 d, 25 nude mice were divided into 5 groups at random and 0.2 mL normal saline solution, 1500 mg/kg DMSO, 500 mg/kg resveratrol, 1000 mg/kg resveratrol, and 1500 mg/kg resveratrol were directly injected beside tumor body respectively 6 times at an interval of 2 d. Then changes of tumor volume (V = (p/6) ×abc) were measured 11 d after injecting drugs and tumor inhibition rate of each group was calculated according to the following formula.
Inhibitory rate{IR} of tumor growth = [C (V1-V0) - T (V1-V0)]/ C (V1-V0)
Where C is control group, T is treated group, V1 is the volume before treatment (mm3), V0 is the volume after treated (mm3).
Tumor samples were cut into 1 mm×1 mm×1 mm sections and fixed in 4% glutaral and immersed with Epon 821, imbedded for 72 h at 60 °C. Cells were prepared into ultrathin section (60 nm) and stained with uranyl acetate and lead citrate. Cell morphology was observed by transmission electron microscopy.
Tumor samples were cryopreservated in liquid nitrogen and cut into 8-μm thick slices. Slices were fixed in ice-cold 80% ethanol for 24 h, treated with proteinase K and 0.3% H2O2, labeled with fluorescein dUTP in a humid box for 1 h at 37 °C. Slices were then combined with POD-horseradish peroxidase, stained with DAB and counterstained with methyl green. Controls received the same management except the labeling of omission of fluorescein dUTP. Cells were visualized with light microscope. The apoptotic index (AI) was calculated as follows: AI = (number of apoptotic cells/total number)×100%.
Tumor samples were cryopreservated in liquid nitrogen, cut into 8-μm thick slices and fixed by acetone. After washed with PBS, slices were incubated in 0.3% H2O2 solution at room temperature for 5 min. Slices were then incubated with anti-bcl-2 or anti-bax monoclonal antibody at a 1:300 dilution at 4 °C overnight. After washed with PBS, the second antibody, biotinylated antirat IgG, was added and cells were incubated at room temperature for 1 h. After washed with PBS, ABC compound was added and incubated at room temperature for 10 min. DAB was used as the chromagen. After 10 min, the brown color signifying the presence of antigens bound to antibodies was detected by light microscopy. Controls were managed as the experimental group except the incubation of primary antibody. The positive rate (PR) was calculated as follows: PR = (number of positive cells/total number)×100%.
Tumor samples were cryopreservated in liquid nitrogen and total RNA was extracted. Concentration of RNA was determined by the absorption at 260 nm. The primers for bcl-2, bax and β-actin were as follows: β-actin (500 bp) 5’ GTGGGGCGCCCCAGGCACCA 3’ (sense); 5’ CTCCTTAATGTCACGCACGATTTC 3’ (anti-sense); bcl-2 (716 bp) 5’ GGAAATATGGCGCACGCT 3’ (sense); 5’ TCACTTGTGGCCCAGAT 3’ (anti-sense); bax (508 bp) 5’ CCAGCTCTGAGCAGATCAT 3’ (sense), 5’ TATCAGCCCATCTTCTTCC 3’ (anti-sense). Polymerse chain reactions were performed in a 50 μL reaction volume. RT-PCR reaction was run in the following conditions: at 94 °C for 7 min, 1 circle; at 94 °C for 1 min, at 72 °C for 1min, 30 circle; at 72 °C for 7 min, 1 circle. Ten μL PCR products was placed onto 15 g/L agarose gel and observed by EB staining using the Gel-Pro analyzer.
Data were analyzed by analysis of variance, and P<0.05 was considered statistically significant.
An inhibitory effect was observed in all therapeutic groups and the inhibition rate of resveratrol at the dose of 500 mg/kg, 1000 mg/kg and 1500 mg/kg was 10.58%, 29.68% and 39.14% respectively (P<0.05 vs the control group, Table 1).
| Group | Number of animals | Volume of tumors (mm3) | Inhibition rate% | ||
| Beginning | Ending | Beginning | Ending | ||
| Control group | |||||
| 0.2 mL saline | 5 | 5 | 20.49±0.99 | 498.73±10.74 | |
| DMSO | |||||
| 1500 mg/kg | 5 | 5 | 20.07±1.24 | 506.17±8.70 | |
| Resveratrol | |||||
| 500 mg/kg | 5 | 5 | 20.44±1.76 | 448.04±6.32a | 10.58 |
| 1000 mg/kg | 5 | 5 | 21.27±1.73 | 357.55±6.34a | 29.68 |
| 1500 mg/kg | 5 | 5 | 21.32±1.72 | 312.39±9.93a | 39.14 |
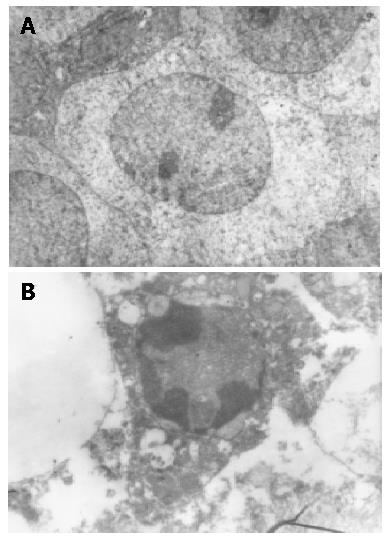
The cells in control groups had normal structures, but some cells in therapeutic groups had apoptotic characteristics including chromatin condensation, chromatin crescent, nucleus fragmentation (Figure 1A, B).
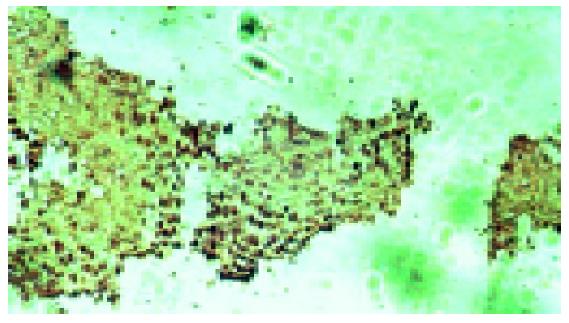
Positive staining was located in nuclei (Figure 2). The apoptosis index of 0.2 mL normal saline solution, 1500 mg/kg DMSO, 500 mg/kg resveratrol, 1000 mg/kg resveratrol, and 1500 mg/kg resveratrol was 13.68±0.37%, 13.8±0.43%, 48.7±1.07%, 56.44±1.39% and 67±0.96%, respectively (P<0.001 vs the control group Table 2).
| Control | DMSO | 500 mg/kg | 1000 mg/kg | 1500 mg/kg | |
| AI (%) | 13.68±0.37 | 13.80±0.43 | 48.70±1.07 | 56.44±1.39 | 67±0.96 |
| F | 0.13 | 1344.25b | 2651.16b | 7984.02b | |
| P | >0.05 | <0.001 | <0.001 | <0.001 |
Positive staining was located in cytoplasm. The positive rate of bcl-2 protein of 0.2 mL normal saline solution, 1500 mg/kg DMSO, 500 mg/kg resveratrol, 1000 mg/kg resveratrol, and 1500 mg/kg resveratrol was 29.48±0.51%, 27.56±1.40%, 11.86±0.97%, 5.7±0.84% and 3.92±0.85% respectively by immunohistochemical staining (P<0.001 vs the control group Table 3).
| Control | DMSO | 500 mg/kg | 1000 mg/kg | 1500 mg/kg | |
| PT(%) | 29.48±0.51 | 27.56±1.40 | 11.86±0.93 | 5.70±0.84 | 3.92±0.85 |
| F | 4.98 | 775.51b | 1879.11b | 1994.65b | |
| P | >0.05 | <0.001 | <0.001 | <0.001 |
Positive staining was located in cytoplasm. The positive rate of bax protein of 0.2 mL normal saline solution, 1500 mg/kg DMSO, 500 mg/kg resveratrol, 1000 mg/kg resveratrol, and 1500 mg/kg resveratrol was 19.34±0.35%, 20.88±0.91%, 40.02±1.20%, 45.72±0.88% and 52.3±1.54% respectively (P<0.001 vs the control group Table 4).
| Control | DMSO | 500 mg/kg | 1000 mg/kg | 1500 mg/kg | |
| PT(%) | 19.34±0.35 | 20.88±0.91 | 40.02±1.20 | 45.72±0.88 | 52.3±1.54 |
| F | 7.48 | 821.11b | 2327.70b | 1298.41b | |
| P | >0.05 | <0.001 | <0.001 | <0.001 |
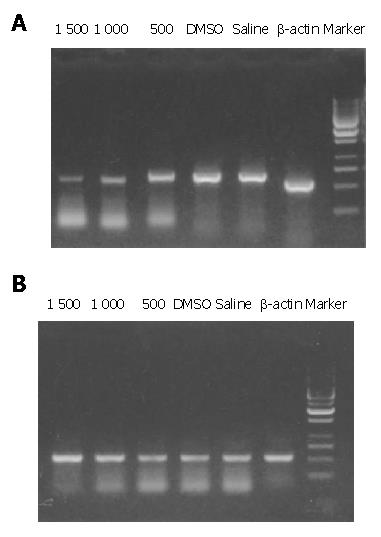
The density of bcl-2 mRNA in 0.2 mL normal saline solution, 1500 mg/kg DMSO, 500 mg/kg resveratrol, 1000 mg/kg resveratrol, and 1500 mg/kg resveratrol decreased progressively and the density of bax mRNA in 0.2 mL normal saline solution, 1500 mg/kg DMSO, 500 mg/kg resveratrol, 1000 mg/kg resveratrol, and 1500 mg/kg increased progressively with elongation of time by RT-PCR (Figure 3A, B ).
Currently, only few chemotherapeutic drugs are effective in the treatment of human primary gastric carcinoma and it is necessary to look for new anti-gastric carcinoma drugs. Resveratrol, a polyphenol has been found in various fruits and vegetables and grapes. The root extract from the weed Polygonum cuspidatum, an important constituent of Chinese folk medicine, is also an ample source of resveratrol[1,2]. Several studys in the past several years have shown that resveratrol has cardioprotective and chemopreventive effects[3-5]. This constituent might account for the reduced risk of coronary heart disease in humans which could be achieved by a moderate wine consumption[6]. Resveratrol was able to inhibit the growth of a wide variety of tumor cells, including leukemic, prostate, breast and hepatic cells[7-11]. The anti-tumor activity of resveratrol might be related to the induction of tumor apoptosis of tumor cells[12-22].
Bcl-2 family plays a crucial role in the control of apoptosis. It has been found that the family includes a number of proteins which have homologous amino acid sequences, including anti-apoptotic members such as bcl-2 and bcl-xL, as well as pro-apoptotic members including bax and bad[23-26]. Overexpression of bax could promote the cell death[27-31]. Conversely, overexpression of antiapoptotic proteins such as Bcl-2 could repress the function of bax[32-36]. Thus, the ratio of bcl-2 /bax was a critical determinant of a cell’s threshold for undergoing apoptosis[37].
We found that resveratrol was able to induce apoptosis in primary gastric cancer in in vitro experiments. This apoptosis might be mediated by down-regulating the expression of apoptosis-regulated gene bcl-2 and up-regulating the expression of apoptosis-regulated gene bax. In this study, we evaluated the effectiveness of apoptosis of gastric cacinoma induced by resveratrol in vivo, investigate the molecular mechanisms further and provide the theoretical and methodological basis for the clinical application of resveratrol.
We observed the inhibitory effect of resveratrol in all therapeutic groups. Cells in control groups had normal structures, but some cells in therapeutic groups had apoptotic characteristics. The apoptosis index of resveratrol at the dose of 500, 1000, and 1500 mg/kg was increased. Expression of bcl-2 of resveratrol at the dose of 500, 1000, and 1500 mg/kg was decreased, but expression of bax was increased. The density of bcl-2 mRNA induced by resveratrol at the dose of 500, 1000, and 1500 mg/kg decreased progressively and the density of bax mRNA increased progressively. The ratio of bcl-2/bax was decreased and triggered the apoptosis of transplanted tumor cells.
Our results demonstrated resveratrol was able to induce the apoptosis of transplanted tumor cells in nude mice. The apoptosis may be mediated by down-regulating the expression of apoptosis-regulated gene bcl-2 and up-regulating the expression of apoptosis-regulated gene bax. Resveratrol may be potentially used as a chemotherapeutic drug in anti-gastric carcinoma chemotherapy.
Edited by Wang XL Proofread by Chen WW
| 1. | Yoon SH, Kim YS, Ghim SY, Song BH, Bae YS. Inhibition of protein kinase CKII activity by resveratrol, a natural compound in red wine and grapes. Life Sci. 2002;71:2145-2152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Gao X, Xu YX, Divine G, Janakiraman N, Chapman RA, Gautam SC. Disparate in vitro and in vivo antileukemic effects of resveratrol, a natural polyphenolic compound found in grapes. J Nutr. 2002;132:2076-2081. [PubMed] |
| 3. | Bhat KP, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Ann N Y Acad Sci. 2002;957:210-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 246] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 4. | Kuwajerwala N, Cifuentes E, Gautam S, Menon M, Barrack ER, Reddy GP. Resveratrol induces prostate cancer cell entry into s phase and inhibits DNA synthesis. Cancer Res. 2002;62:2488-2492. [PubMed] |
| 5. | Joe AK, Liu H, Suzui M, Vural ME, Xiao D, Weinstein IB. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin Cancer Res. 2002;8:893-903. [PubMed] |
| 6. | Wang Z, Zou J, Huang Y, Cao K, Xu Y, Wu JM. Effect of resveratrol on platelet aggregation in vivo and in vitro. Chin Med J (Engl). 2002;115:378-380. [PubMed] |
| 7. | Ferry-Dumazet H, Garnier O, Mamani-Matsuda M, Vercauteren J, Belloc F, Billiard C, Dupouy M, Thiolat D, Kolb JP, Marit G. Resveratrol inhibits the growth and induces the apoptosis of both normal and leukemic hematopoietic cells. Carcinogenesis. 2002;23:1327-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Kampa M, Hatzoglou A, Notas G, Damianaki A, Bakogeorgou E, Gemetzi C, Kouroumalis E, Martin PM, Castanas E. Wine antioxidant polyphenols inhibit the proliferation of human prostate cancer cell lines. Nutr Cancer. 2000;37:223-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 152] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Bove K, Lincoln DW, Tsan MF. Effect of resveratrol on growth of 4T1 breast cancer cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;291:1001-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Tian XM, Zhang ZX. The anticancer activity of resveratrol on implanted tumor of HepG2 in nude mice. Shijie Huaren Xiaohua Zazhi. 2001;9:161-164. |
| 11. | Sun ZJ, Pan CE, Liu HS, Wang GJ. Anti-hepatoma activity of resveratrol in vitro. World J Gastroenterol. 2002;8:79-81. [PubMed] |
| 12. | Pervaiz S. Resveratrol--from the bottle to the bedside? Leuk Lymphoma. 2001;40:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Dörrie J, Gerauer H, Wachter Y, Zunino SJ. Resveratrol induces extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells. Cancer Res. 2001;61:4731-4739. [PubMed] |
| 14. | She QB, Bode AM, Ma WY, Chen NY, Dong Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. 2001;61:1604-1610. [PubMed] |
| 15. | Tsan MF, White JE, Maheshwari JG, Bremner TA, Sacco J. Resveratrol induces Fas signalling-independent apoptosis in THP-1 human monocytic leukaemia cells. Br J Haematol. 2000;109:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Szende B, Tyihák E, Király-Véghely Z. Dose-dependent effect of resveratrol on proliferation and apoptosis in endothelial and tumor cell cultures. Exp Mol Med. 2000;32:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Bernhard D, Tinhofer I, Tonko M, Hübl H, Ausserlechner MJ, Greil R, Kofler R, Csordas A. Resveratrol causes arrest in the S-phase prior to Fas-independent apoptosis in CEM-C7H2 acute leukemia cells. Cell Death Differ. 2000;7:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Mouria M, Gukovskaya AS, Jung Y, Buechler P, Hines OJ, Reber HA, Pandol SJ. Food-derived polyphenols inhibit pancreatic cancer growth through mitochondrial cytochrome C release and apoptosis. Int J Cancer. 2002;98:761-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 202] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Shih A, Davis FB, Lin HY, Davis PJ. Resveratrol induces apoptosis in thyroid cancer cell lines via a MAPK- and p53-dependent mechanism. J Clin Endocrinol Metab. 2002;87:1223-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Mahyar-Roemer M, Katsen A, Mestres P, Roemer K. Resveratrol induces colon tumor cell apoptosis independently of p53 and precede by epithelial differentiation, mitochondrial proliferation and membrane potential collapse. Int J Cancer. 2001;94:615-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Lin HY, Shih A, Davis FB, Tang HY, Martino LJ, Bennett JA, Davis PJ. Resveratrol induced serine phosphorylation of p53 causes apoptosis in a mutant p53 prostate cancer cell line. J Urol. 2002;168:748-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | She QB, Huang C, Zhang Y, Dong Z. Involvement of c-jun NH(2)-terminal kinases in resveratrol-induced activation of p53 and apoptosis. Mol Carcinog. 2002;33:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 316] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 24. | van der Woude CJ, Jansen PL, Tiebosch AT, Beuving A, Homan M, Kleibeuker JH, Moshage H. Expression of apoptosis-related proteins in Barrett's metaplasia-dysplasia-carcinoma sequence: a switch to a more resistant phenotype. Hum Pathol. 2002;33:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Panaretakis T, Pokrovskaja K, Shoshan MC, Grandér D. Activation of Bak, Bax, and BH3-only proteins in the apoptotic response to doxorubicin. J Biol Chem. 2002;277:44317-44326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Bellosillo B, Villamor N, López-Guillermo A, Marcé S, Bosch F, Campo E, Montserrat E, Colomer D. Spontaneous and drug-induced apoptosis is mediated by conformational changes of Bax and Bak in B-cell chronic lymphocytic leukemia. Blood. 2002;100:1810-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Matter-Reissmann UB, Forte P, Schneider MK, Filgueira L, Groscurth P, Seebach JD. Xenogeneic human NK cytotoxicity against porcine endothelial cells is perforin/granzyme B dependent and not inhibited by Bcl-2 overexpression. Xenotransplantation. 2002;9:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Lanzi C, Cassinelli G, Cuccuru G, Supino R, Zuco V, Ferlini C, Scambia G, Zunino F. Cell cycle checkpoint efficiency and cellular response to paclitaxel in prostate cancer cells. Prostate. 2001;48:254-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Mertens HJ, Heineman MJ, Evers JL. The expression of apoptosis-related proteins Bcl-2 and Ki67 in endometrium of ovulatory menstrual cycles. Gynecol Obstet Invest. 2002;53:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Mehta U, Kang BP, Bansal G, Bansal MP. Studies of apoptosis and bcl-2 in experimental atherosclerosis in rabbit and influence of selenium supplementation. Gen Physiol Biophys. 2002;21:15-29. [PubMed] |
| 31. | Chang WK, Yang KD, Chuang H, Jan JT, Shaio MF. Glutamine protects activated human T cells from apoptosis by up-regulating glutathione and Bcl-2 levels. Clin Immunol. 2002;104:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Chen GG, Lai PB, Hu X, Lam IK, Chak EC, Chun YS, Lau WY. Negative correlation between the ratio of Bax to Bcl-2 and the size of tumor treated by culture supernatants from Kupffer cells. Clin Exp Metastasis. 2002;19:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Usuda J, Chiu SM, Azizuddin K, Xue LY, Lam M, Nieminen AL, Oleinick NL. Promotion of photodynamic therapy-induced apoptosis by the mitochondrial protein Smac/DIABLO: dependence on Bax. Photochem Photobiol. 2002;76:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 34. | Sun F, Akazawa S, Sugahara K, Kamihira S, Kawasaki E, Eguchi K, Koji T. Apoptosis in normal rat embryo tissues during early organogenesis: the possible involvement of Bax and Bcl-2. Arch Histol Cytol. 2002;65:145-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Jang MH, Shin MC, Shin HS, Kim KH, Park HJ, Kim EH, Kim CJ. Alcohol induces apoptosis in TM3 mouse Leydig cells via bax-dependent caspase-3 activation. Eur J Pharmacol. 2002;449:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Tilli CM, Stavast-Koey AJ, Ramaekers FC, Neumann HA. Bax expression and growth behavior of basal cell carcinomas. J Cutan Pathol. 2002;29:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Pettersson F, Dalgleish AG, Bissonnette RP, Colston KW. Retinoids cause apoptosis in pancreatic cancer cells via activation of RAR-gamma and altered expression of Bcl-2/Bax. Br J Cancer. 2002;87:555-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |