Published online Jan 14, 2005. doi: 10.3748/wjg.v11.i2.275
Revised: March 18, 2004
Accepted: May 13, 2004
Published online: January 14, 2005
AIM: To investigate the relationship between mutations of rearranged during transfection (RET) proto-oncogene and Chinese patients with Hirschsprung’s disease (HD), and to elucidate the genetic mechanism of familial HD patient at the molecular level.
METHODS: Genomic DNA was extracted from venous blood of probands and their relatives in two genealogies. Polymerase chain reaction (PCR) products, which were amplified using specific primers (RET, exons 11, 13, 15 and 17), were electrophoresed to analyze the single-strand conformational polymorphism (SSCP) patterns. The positive amplified products were sequenced. Forty-eight sporadic HD patients and 30 normal children were screened for mutations of RET proto-oncogene simultaneously.
RESULTS: Three cases with HD in one family were found to have a G heterozygous insertion at nucleotide 18974 in exon 13 of RET cDNA (18974insG), which resulted in a frameshift mutation. In another family, a heterozygosity for T to G transition at nucleotide 18888 in the same exon which resulted in a synonymous mutation of Leu at codon 745 was detected in the proband and his father. Eight RET mutations were confirmed in 48 sporadic HD patients.
CONCLUSION: Mutations of RET proto-oncogene may play an important role in the pathogenesis of Chinese patients with HD. Detection of mutated RET proto-oncogene carriers may be used for genetic counseling of potential risk for HD in the affected families.
- Citation: Guan T, Li JC, Li MJ, Tou JF. Polymerase chain reaction-single strand conformational polymorphism analysis of rearranged during transfection proto-oncogene in Chinese familial hirschsprung’s disease. World J Gastroenterol 2005; 11(2): 275-279
- URL: https://www.wjgnet.com/1007-9327/full/v11/i2/275.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i2.275
Hirschsprung’s disease (HD) is a common congenital malformation affecting 1 in 5000 live births. Absence of parasympathetic neuronal ganglia in the hindgut results in poor coordination of peristaltic movement and varying degrees of constipation[1-6]. Although the pathogenesis of HD has not been fully understood, the familial occurrence with an increased risk for siblings as well as an uneven gender distribution indicates a genetic cause of the disease. RET proto-oncogene, encoding a 1114 residue receptor tyrosine kinase, is thought to be essential for neurogenesis of the enteric nervous system[7-11]. Through detailed examination more than 60 missense mutations or deletions have been found along the whole coding sequence of RET in HD[8,12-21]. It was reported that RET mutations were identified in approximately 50% of familial cases of HD and in 10-20% of sporadic cases abroad[7,22-24]. However, few studies on HD in Chinese population are reported.
In this study, we performed genetic analysis of exons 11, 13, 15 and 17 of RET proto-oncogene in two HD genealogies by using polymerase chain reaction-single strand conformation polymorphism (PCR-SSCP). In order to further investigate the pathogenic mechanism of HD, 48 sporadic HD patients and 30 normal children were also screened by SSCP.
Probands of two HD families were collected at Zhejiang Children’s Hospital. The criteria[25] for HD were absence of enteric plexuses plus the aganglionic tract and increased acetylcholinesterase staining of nerve fibers. Forty-eight Chinese patients with sporadic HD (35 males and 13 females) and 30 normal children were enrolled in this study. Genomic DNA was isolated from peripheral white blood cells according to standard procedures[26].
Primers were designed to amplify the exons and flanking intronic sequences (Table 1). PCR amplification was performed in a reaction volume of 50 μL containing 200 ng of genomic DNA, 10 mmol/L Tris-HCl (pH 8.4), 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.5 μmol/L each of two fragment-specific primers, 100 μmol/L each of dATP, dGTP, dTTP and dCTP, and 2 units of Taq DNA polymerase. The template-melting step of PCR amplification was at 94 °C for 5 min, followed by 30 cycles of serial incubation at 94 °C for 50, at 58 °C to 62 °C for 50 min, and at 72 °C for 10 min. PCR products were electrophoresed on 1% agarose gel and 100-bp ladder markers were used to compare the size of amplified fragments.
| Exon | Sequence (5'→3') | Annealing t (°C) | Product size (bp) | |
| 11 | F: ACACCACCCCCACCCACAGAT | 62 | 273 | |
| R: AAGCTTGAAGGCATCCACGG | ||||
| 13 | F: GACCTGGTATGGTCATGGA | 58 | 253 | |
| R: AAGAGGGAGAACAGGGCTGTA | ||||
| 15 | F: GACTCGTGCTATTTTTCCTAC | 60 | 234 | |
| R: TATCTTTCCTAGGCTTCCC | ||||
| 17 | F: CCCCACTAGATGTATAAGGG | 59 | 232 | |
| R: TCACTGGTCCTTTCACTCTCT |
SSCP analysis of fragments was performed on a mini electrophoresis unit (Bio-Rad Company, USA). A total of 10 μL of the PCR product was diluted with 10 μL of sample buffer containing 900 mL/L formamide, 0.5 g/L bromphenol blue dye and 0.5 g/L xylene cyanol. The samples were heated at 100 °C for 8 min, transferred into an ice-cold water bath for 3 min, and analyzed by 80 g/L polyacryl amide gel electrophoresis (PAGE) in 45 mmol/L-Tris-borate (pH 8.0)/1 mmol/L-EDTA (TBE) buffer under 13 v/cm at 10 °C.
Gels were fixed in 100 mL/L alcohol for 10 min and oxidized in 100 mL/L nitric acid. After 3 min, gels were washed for 1 min with double distilled water, then stained in 2 g/L silver nitric acid for 5 min, and again washed for 1 min with double distilled water. The gels showed appropriate color in 15 g/L anhydrous sodium carbonate and 4 mL/L formalin and ended reducing response by 7.5 mL/L glacial acetic acid. Subsequently, gels were washed with double distilled water. At last, the results of silver staining were analyzed and photographed.
Abnormal PCR products screened by SSCP were cut from gel and purified according to Viogene kit manufacturer’s instructions. Sequence analysis was carried out with a PE377 automated sequencer.
The increment of all DNA samples from HD patients was a single strand with the same length as normal controls, indicating that a large fragment insertion and deletion did not exist in the region in exons 11, 13, 15 and 17 of RET proto-oncogene among HD patients.
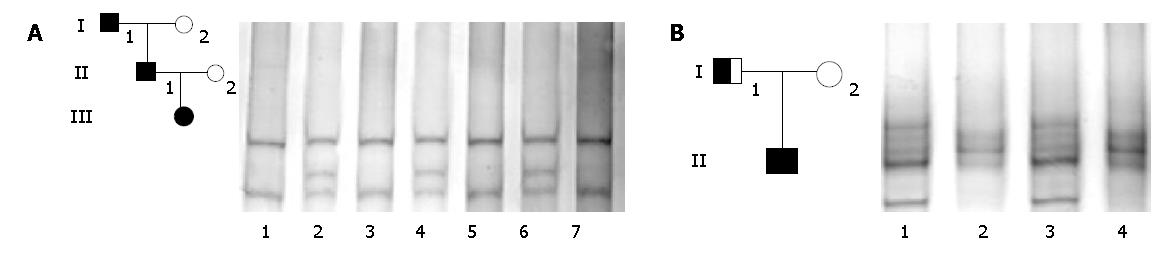
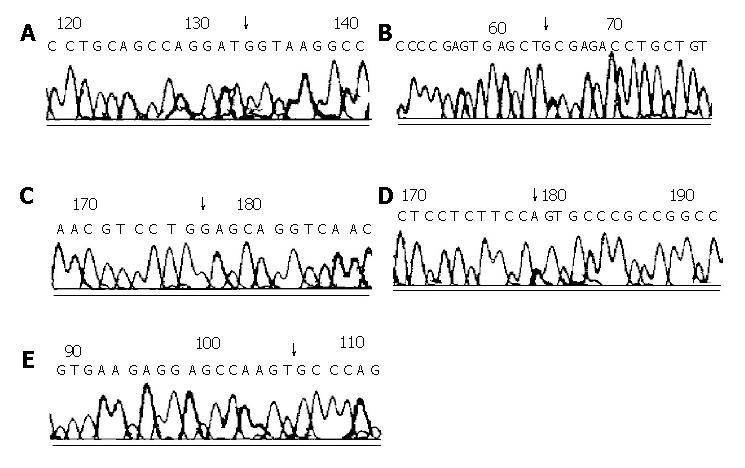
HD was observed in 3 generations in patients III, II 1 and I 1 (Figure 1A). The female proband (III) carried a large aganglionic region in the colon that was removed by surgery at the age of 1 year. Her farther also developed the long aganglionic form of HD and underwent surgery at age of two years. The male I 1 in the first ge neration suffered from chronic obstipation. We analyzed the genomic DNA of the 5 familial members for mutations in exons 11, 13, 15 and 17 of RET proto-oncogene. All three HD patients demonstrated abnormal SSCP patterns in exon 13 (Figure 1A). DNA sequencing revealed a G heterozygous insertion at nucleotide 18 974 (18 974insG) in exon 13 of RET proto-oncogene encoding the intracellular part of RET receptors (Figure 2A), which resulted in a frameshift mutation. We failed to detect any additional mutation in RET proto-oncogene in DNA of the unaffected members in this family.
In this family, one son (II) suffered from HD and was operated for the disease at the age of 3 mo. The aganglionosis in II was confined to the rectosigmoid colon. On SSCP screening of RET proto-oncogene, II and his father whose phenotype was normal showed distinct aberrant band patterns in exon 13 (Figure 1B). DNA sequencing showed a T to G transition in codon 745. This nucleotide was exchanged from CTT to CTG; however, it was mute and did not alter the tyrosine in this position. This synonymous mutation was found to be heterozygous (Figure 2B). In DNA of the unaffected mother, the nucleotide sequence at codon 745 was unaltered.
Mutation changes were detected in 8 of 48 sporadic HD patients in RET proto-oncogene. But in 30 normal individuals no mutation change was observed. This mutation rate in the gene was estimated to be 16.7% (8/48). DNA sequence analysis showed that there were 5 patterns of nucleotide changes: A G to A substitution was observed at codon 667 (G667S) in exon 11 encoding the tyrosine kinase domain of the receptor in 3 samples (Figure 2D). Another a G to A substitution was identified at codon 916 (Q916Q) in exon 15 in one patient, but it was a synonymous mutation (Figure 2E). In exon 13, a A to G transition was found at colon 756 (K756E) in one patient (Figure 2C). A T to G mutation, although it seemed to be a silent mutation, was revealed in codon 745 (L745L) in another patient (Figure 2C). A G heterozygous insertion at nucleotide 18 974 (18 974insG) was found in 2 patients. Among the 8 patients with RET mutations, 5 were patients with sporadic long-segment (aganglionosis confined to the transverse colon) HD and 3 were patients with sporadic short-segment (aganglionosis confined to the rectosigmoid colon) HD. All these mutation changes were identified to be heterozygous, and are summarized in Table 2.
| Case | Sex | Range of aganglionic segment | Exon | Nucleotide change | Amino acid change | Mutation types |
| 1 | M | Long-segment | 11 | G15165→A | G667S | Missense mutation |
| 2 | M | Short-segment | 11 | G15165→A | G667S | Missense mutation |
| 3 | F | Long-segment | 11 | G15165→A | G667S | Missense mutation |
| 4 | M | Long-segment | 13 | T18888→G | L745L | Silent mutation |
| 5 | M | Long-segment | 13 | A18919→G | K756E | Missense mutation |
| 6 | M | Long-segment | 13 | 18974insG | - - - - | Frameshift mutation |
| 7 | M | Short-segment | 13 | 18974insG | - - - - | Frameshift mutation |
| 8 | M | Short-segment | 15 | G20692→A | Q916Q | Silent mutation |
The human RET proto-oncogene has been mapped to chromosome band 10q11.2 and comprises 20 exons with a length of about 80 kb[27-30]. Receptor tyrosine kinase encoded by RET proto-oncogene consists of an intracellular tyrosine kinase domain, a transmembrane domain and an extracellular domain which includes a “cadherin-like” region. Receptor tyrosine kinases generally function as ligand-dependent dimmers, which phosphorylate “second messenger” proteins in the cytoplasm, and they are commonly associated with the regulation of cell growth and differentiation, development of normal nerves, and expressed in ganglionic source cells, such as neurogenic ganglia and ganglia of peripheral nerve system, neuroendocrine cells, epidermic pigment cells, etc.[9]. RET proto-oncogene is controlled by a promoter harboring four randomly repeated 5’-CG-3’ sequences that are part of a 5’-CG-3’ dense region in proximity to the transcriptional start site[31]. Ninety-five unmethylated 5’-CG-3’ dinucleotides are located in a region of about 900 bp in which the majority lie within the putative promoter segment. During embryogenesis, RET proto-oncogene expression is regulated in a temporally and spatially defined pattern. In adults, RET mRNA transcripts could be detected exclusively in substantia nigra, adrenal medulla, cerebellum and other parts of the brain[31-35]. RET proto-oncogene alterations as disease-causing mutations have been demonstrated in five different disease entities: HD, papillary thyroid carcinoma, and three types of inherited cancer syndromes, multiple endocrine neoplasia (MEN) 2A, MEN 2B and familial modullary thyroid carcinoma (FMTC)[21,36-42].
To date, only 15-20% of all HD cases were reported to be of familial origin, and 80% of these were reported to be caused by mutations in either RET proto-oncogene or other candidate genes[1,6]. Recently, Munnes et al[32] identified and analyzed six HD families living in Germany by PCR-DNA sequencing. They observed mutations at codon 609 (C609R) in one out of 6 cysteine residues encoded in exon 10 of RET proto-oncogene in one family with a joint occurrence of HD and FMTC. The other 2 patterns of RET mutations (R77C, Y204Y) were found in 2 different families. Up to now, mutation analysis of candidate genes has not been reported in Chinese HD pedigree[43]. In this study, we have found that three HD cases in one Chinese HD family carried a frame-shift mutation (18974insG) in exon 13 of RET proto-oncogene. This novel mutation could alter the amino acid sequence in the tyrosine kinase domain of the RET receptor, and the signal conduction was obstructed, thereby finally causing HD. Therefore, in this family, RET mutation analysis enabled us to concentrate our clinical efforts on family members at high risk for HD. In mutated RET proto-oncogene carriers, therapeutic planning could be made based on the natural history of this disease. Genetic information on RET genotye-phenotypic correlation might be used for genetic counseling of potential risk for HD. In another family, the sick child and his father had the same heterozygous silent mutation (L745L) in exon 13 of RET proto-oncogene. However, the father’s phenotype was normal, and penetrance of HD was incomplete. It is suggested that phenotypic expression of this disease might depend not only on the RET mutation pattern but also on other genetic or environmental determinants, although it has a genetic tendency.
In the 48 sporadic HD patients, we detected 6 disease-causing mutations and 2 silent mutations. These mutations were revealed to be heterozygous. Studies have reported that only a half quantity of RET proto-oncogene mutations is likely to cause HD in humans[44-46], which was also confirmed by our finding. We observed that 2 of the 6 disease-causing mutations were detected in HD patients with short-segment aganglionosis suggesting that mutations of RET proto-oncogene are associated not only with long-segment HD, but also with sporadically occurring short-segment HD in Chinese population with this disease.
Our results suggest that mutations of RET proto-oncogene may play an important role in the pathogenesis of Chinese patients with HD. Mutation analysis of the gene may provide an additive diagnostic value for this disease, especially familial cases. However, the low mutation rate and no spot of mutations in this gene indicate that other genes and microenvironmental factors can be involved in the development of HD. Further investigation is necessary to elucidate the pathogenesis of this disease.
Edited by Kumar M and Wang XL
| 1. | Nogueira A, Campos M, Soares-Oliveira M, Estevão-Costa J, Silva P, Carneiro F, Carvalho JL. Histochemical and immunohistochemical study of the intrinsic innervation in colonic dysganglionosis. Pediatr Surg Int. 2001;17:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Yoneda A, Wang Y, O'Briain DS, Puri P. Cell-adhesion molecules and fibroblast growth factor signalling in Hirschsprung's disease. Pediatr Surg Int. 2001;17:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Li JC, Mi KH, Zhou JL, Busch L, Kuhnel W. The development of colon innervation in trisomy 16 mice and Hirschsprung's disease. World J Gastroenterol. 2001;7:16-21. [PubMed] |
| 4. | Won KJ, Torihashi S, Mitsui-Saito M, Hori M, Sato K, Suzuki T, Ozaki H, Karaki H. Increased smooth muscle contractility of intestine in the genetic null of the endothelin ETB receptor: a rat model for long segment Hirschsprung's disease. Gut. 2002;50:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Amiel J, Lyonnet S. Hirschsprung disease, associated syndromes, and genetics: a review. J Med Genet. 2001;38:729-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 286] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 6. | Rice J, Doggett B, Sweetser DA, Yanagisawa H, Yanagisawa M, Kapur RP. Transgenic rescue of aganglionosis and piebaldism in lethal spotted mice. Dev Dyn. 2000;217:120-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Iwashita T, Kurokawa K, Qiao S, Murakami H, Asai N, Kawai K, Hashimoto M, Watanabe T, Ichihara M, Takahashi M. Functional analysis of RET with Hirschsprung mutations affecting its kinase domain. Gastroenterology. 2001;121:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Griseri P, Pesce B, Patrone G, Osinga J, Puppo F, Sancandi M, Hofstra R, Romeo G, Ravazzolo R, Devoto M. A rare haplotype of the RET proto-oncogene is a risk-modifying allele in hirschsprung disease. Am J Hum Genet. 2002;71:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Manié S, Santoro M, Fusco A, Billaud M. The RET receptor: function in development and dysfunction in congenital malformation. Trends Genet. 2001;17:580-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 205] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Japón MA, Urbano AG, Sáez C, Segura DI, Cerro AL, Diéguez C, Alvarez CV. Glial-derived neurotropic factor and RET gene expression in normal human anterior pituitary cell types and in pituitary tumors. J Clin Endocrinol Metab. 2002;87:1879-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Gershon MD. Lessons from genetically engineered animal models. II. Disorders of enteric neuronal development: insights from transgenic mice. Am J Physiol. 1999;277:G262-G267. [PubMed] |
| 12. | Onochie CI, Korngut LM, Vanhorne JB, Myers SM, Michaud D, Mulligan LM. Characterisation of the human GFRalpha-3 locus and investigation of the gene in Hirschsprung disease. J Med Genet. 2000;37:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Inoue K, Shimotake T, Iwai N. Mutational analysis of RET/GDNF/NTN genes in children with total colonic aganglionosis with small bowel involvement. Am J Med Genet. 2000;93:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Gath R, Goessling A, Keller KM, Koletzko S, Coerdt W, Müntefering H, Wirth S, Hofstra RM, Mulligan L, Eng C. Analysis of the RET, GDNF, EDN3, and EDNRB genes in patients with intestinal neuronal dysplasia and Hirschsprung disease. Gut. 2001;48:671-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Julies MG, Moore SW, Kotze MJ, du Plessis L. Novel RET mutations in Hirschsprung's disease patients from the diverse South African population. Eur J Hum Genet. 2001;9:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Inoue K, Shimotake T, Tomiyama H, Iwai N. Mutational analysis of the RET and GDNF gene in children with hypoganglionosis. Eur J Pediatr Surg. 2001;11:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Shimotake T, Go S, Inoue K, Tomiyama H, Iwai N. A homozygous missense mutation in the tyrosine E kinase domain of the RET proto-oncogene in an infant with total intestinal aganglionosis. Am J Gastroenterol. 2001;96:1286-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Hofstra RM, Wu Y, Stulp RP, Elfferich P, Osinga J, Maas SM, Siderius L, Brooks AS, vd Ende JJ, Heydendael VM. RET and GDNF gene scanning in Hirschsprung patients using two dual denaturing gel systems. Hum Mutat. 2000;15:418-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Sancandi M, Ceccherini I, Costa M, Fava M, Chen B, Wu Y, Hofstra R, Laurie T, Griffths M, Burge D. Incidence of RET mutations in patients with Hirschsprung's disease. J Pediatr Surg. 2000;35:139-142; discussion 142-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Fitze G, Cramer J, Ziegler A, Schierz M, Schreiber M, Kuhlisch E, Roesner D, Schackert HK. Association between c135G/A genotype and RET proto-oncogene germline mutations and phenotype of Hirschsprung's disease. Lancet. 2002;359:1200-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Pigny P, Bauters C, Wemeau JL, Houcke ML, Crepin M, Caron P, Giraud S, Calender A, Buisine MP, Kerckaert JP. A novel 9-base pair duplication in RET exon 8 in familial medullary thyroid carcinoma. J Clin Endocrinol Metab. 1999;84:1700-1704. [PubMed] |
| 22. | Borrego S, Ruiz A, Saez ME, Gimm O, Gao X, López-Alonso M, Hernández A, Wright FA, Antiñolo G, Eng C. RET genotypes comprising specific haplotypes of polymorphic variants predispose to isolated Hirschsprung disease. J Med Genet. 2000;37:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Martucciello G, Ceccherini I, Lerone M, Jasonni V. Pathogenesis of Hirschsprung's disease. J Pediatr Surg. 2000;35:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Bolk S, Pelet A, Hofstra RM, Angrist M, Salomon R, Croaker D, Buys CH, Lyonnet S, Chakravarti A. A human model for multigenic inheritance: phenotypic expression in Hirschsprung disease requires both the RET gene and a new 9q31 locus. Proc Natl Acad Sci USA. 2000;97:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Khan AR, Vujanic GM, Huddart S. The constipated child: how likely is Hirschsprung's disease? Pediatr Surg Int. 2003;19:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Pepiński W, Sołtyszewski I, Janica J, Skawrońska M, Koc-Zórawska E. Comparison of five commercial kits for DNA extraction from human blood, saliva and muscle samples. Rocz Akad Med Bialymst. 2002;47:270-275. [PubMed] |
| 27. | Lesueur F, Corbex M, McKay JD, Lima J, Soares P, Griseri P, Burgess J, Ceccherini I, Landolfi S, Papotti M. Specific haplotypes of the RET proto-oncogene are over-represented in patients with sporadic papillary thyroid carcinoma. J Med Genet. 2002;39:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Melillo RM, Santoro M, Ong SH, Billaud M, Fusco A, Hadari YR, Schlessinger J, Lax I. Docking protein FRS2 links the protein tyrosine kinase RET and its oncogenic forms with the mitogen-activated protein kinase signaling cascade. Mol Cell Biol. 2001;21:4177-4187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Griseri P, Sancandi M, Patrone G, Bocciardi R, Hofstra R, Ravazzolo R, Devoto M, Romeo G, Ceccherini I. A single-nucleotide polymorphic variant of the RET proto-oncogene is underrepresented in sporadic Hirschsprung disease. Eur J Hum Genet. 2000;8:721-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Hansford JR, Mulligan LM. Multiple endocrine neoplasia type 2 and RET: from neoplasia to neurogenesis. J Med Genet. 2000;37:817-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 153] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Munnes M, Patrone G, Schmitz B, Romeo G, Doerfler W. A 5'-CG-3'-rich region in the promoter of the transcriptionally frequently silenced RET protooncogene lacks methylated cytidine residues. Oncogene. 1998;17:2573-2583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Munnes M, Fanaei S, Schmitz B, Muiznieks I, Holschneider AM, Doerfler W. Familial form of hirschsprung disease: nucleotide sequence studies reveal point mutations in the RET proto-oncogene in two of six families but not in other candidate genes. Am J Med Genet. 2000;94:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 33. | Parisi MA, Kapur RP. Genetics of Hirschsprung disease. Curr Opin Pediatr. 2000;12:610-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 140] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Amiel J, Salomon R, Attié-Bitach T, Touraine R, Steffann J, Pelet A, Nihoul-Fékété C, Vekemans M, Munnich A, Lyonnet S. Molecular genetics of Hirschsprung disease: a model of multigenic neurocristopathy. J Soc Biol. 2000;194:125-128. [PubMed] |
| 35. | Matera I, De Miguel-Rodríguez M, Fernández-Santos JM, Santamaria G, Puliti A, Ravazzolo R, Romeo G, Galera-Davidson H, Ceccherini I. cDNA sequence and genomic structure of the rat RET proto-oncogene. DNA Seq. 2000;11:405-417. [PubMed] |
| 36. | Sakai T, Nirasawa Y, Itoh Y, Wakizaka A. Japanese patients with sporadic Hirschsprung: mutation analysis of the receptor tyrosine kinase proto-oncogene, endothelin-B receptor, endothelin-3, glial cell line-derived neurotrophic factor and neurturin genes: a comparison with similar studies. Eur J Pediatr. 2000;159:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | De Vita G, Melillo RM, Carlomagno F, Visconti R, Castellone MD, Bellacosa A, Billaud M, Fusco A, Tsichlis PN, Santoro M. Tyrosine 1062 of RET-MEN2A mediates activation of Akt (protein kinase B) and mitogen-activated protein kinase pathways leading to PC12 cell survival. Cancer Res. 2000;60:3727-3731. [PubMed] |
| 38. | Fugazzola L, Cerutti N, Mannavola D, Ghilardi G, Alberti L, Romoli R, Beck-Peccoz P. Multigenerational familial medullary thyroid cancer (FMTC): evidence for FMTC phenocopies and association with papillary thyroid cancer. Clin Endocrinol (Oxf). 2002;56:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Wohllk N, Becker P, Youlton R, Cote GJ, Gagel RF. Germline mutations of the ret proto-oncogene in Chilean patients with hereditary and sporadic medullary thyroid carcinoma. Rev Med Chil. 2001;129:713-718. [PubMed] |
| 40. | Loré F, Talidis F, Di Cairano G, Renieri A. Multiple endocrine neoplasia type 2 syndromes may be associated with renal malformations. J Intern Med. 2001;250:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Takano T, Miyauchi A, Yoshida H, Hasegawa Y, Kuma K, Amino N. Large-scale analysis of mutations in RET exon 16 in sporadic medullary thyroid carcinomas in Japan. Jpn J Cancer Res. 2001;92:645-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 42. | Chiefari E, Chiarella R, Crocetti U, Tardio B, Arturi F, Russo D, Trischitta V, Filetti S, Zingrillo M. A large family with hereditary MTC: role of RET genetic analysis in differential diagnosis between MEN 2A and FMTC. Horm Metab Res. 2001;33:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 43. | Garcia-Barceló M, Sham MH, Lee WS, Lui VC, Chen BL, Wong KK, Wong JS, Tam PK. Highly recurrent RET mutations and novel mutations in genes of the receptor tyrosine kinase and endothelin receptor B pathways in Chinese patients with sporadic Hirschsprung disease. Clin Chem. 2004;50:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Sakai T, Wakizaka A, Nirasawa Y. Congenital central hypoventilation syndrome associated with Hirschsprung's disease: mutation analysis of the RET and endothelin-signaling pathways. Eur J Pediatr Surg. 2001;11:335-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Pasini B, Rossi R, Ambrosio MR, Zatelli MC, Gullo M, Gobbo M, Collini P, Aiello A, Pansini G, Trasforini G. RET mutation profile and variable clinical manifestations in a family with multiple endocrine neoplasia type 2A and Hirschsprung's disease. Surgery. 2002;131:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Mograbi B, Bocciardi R, Bourget I, Juhel T, Farahi-Far D, Romeo G, Ceccherini I, Rossi B. The sensitivity of activated Cys Ret mutants to glial cell line-derived neurotrophic factor is mandatory to rescue neuroectodermic cells from apoptosis. Mol Cell Biol. 2001;21:6719-6730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |