Published online Jan 14, 2005. doi: 10.3748/wjg.v11.i2.260
Revised: April 12, 2004
Accepted: June 7, 2004
Published online: January 14, 2005
AIM: Malignant gastrointestinal stromal tumors (GISTs) are rare. Tumors larger than 10 cm tend to recur earlier: the larger the volume of the tumor, the worse the prognosis. We hypothesized that treatment with imatinib mesylate (Gleevec; STI-571), a c-kit tyrosine kinase inhibitor, as palliative therapy would prolong the survival of patients with recurrent giant malignant GISTs after resection.
METHODS: We performed a retrospective analysis of the effects of resection on patients with giant GISTs (>10 cm in diameter) to determine the overall survival and recurrence rates. Twenty-three patients diagnosed with giant GISTs were included from June 1996 to December 2003. STI-571 was not available until January 2000. After that time, 9 patients received this drug. The factors of age, sex, tumor location, histological surgical margin, and STI-571, tumor size changes and drug side effects were reviewed. We compared the survival rate to determine the prognostic factors and the effects of STI-571 on patients with recurrent malignant gastrointestinal stromal tumor.
RESULTS: The positive surgical margin group had a significantly higher recurrence rate than the negative margin group (P = 0.012). A negative surgical margin and palliative treatment with STI-571 were significant prognostic variables (Log-rank test, P<0.05). Age, sex and tumor location were not significant prognostic variables. The 5-year survival rate of the surgical margin free patients was 80% and the 2-year survival rate of the surgical margin positive patients was 28%. The 5-year survival rate was 80% for the patients given STI-571 and 30% for the patients not given STI-571. The use of STI-571 gave a significant tumor shrinkage (6/9) rate in patients with giant GIST recurrence after resection.
CONCLUSION: A negative surgical margin and the use of STI-571 after surgical resection were good prognostic indicators. Achieving a tumor-free surgical margin is still the best primary treatment for patients with such tumors. If STI-571 is used immediately when the surgical margin is positive and the tumor recurs after resection, then the prognosis of patients with giant GISTs can be improved.
- Citation: Chen TW, Liu HD, Shyu RY, Yu JC, Shih ML, Chang TM, Hsieh CB. Giant malignant gastrointestinal stromal tumors: Recurrence and effects of treatment with STI-571. World J Gastroenterol 2005; 11(2): 260-263
- URL: https://www.wjgnet.com/1007-9327/full/v11/i2/260.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i2.260
Gastrointestinal stromal tumors (GISTs) are specific mesenchymal tumors that can occur through the entire gastrointestinal tract, and in the omentum and mesentery. They range from small, benign, incidentally detected nodules to large malignant tumors. GISTs have been proposed to originate from the interstitial cells of Cajal, which are intestinal pacemakers[1]. They are derived from the myeloid stem cells, being positive for the CD34 antigen in 52-72% of cases[2], and are frequently marked by the presence of the c-kit proto-oncogene (85-94%)[3]. Cajal cells have the characteristics of both smooth muscle and neural cells, and neoplastic Cajal cells might preferentially express one, both, or neither of these features, thus explaining the variant forms of GISTs.
Surgical removal remains the only curative treatment for patients with GISTs[4]. Tumor size[5,6], mitotic index[6], anatomic location[7], tumor rupture and disease-free interval[8] are the classic characteristics used to predict the clinical course of patients who undergo complete gross resection. DeMatteo analyzed 200 patients with malignant GISTs and found that patients with tumors larger than 10 cm had shorter disease-free intervals (tumor recurrence)[5]. Tumor size was a predictor of tumor recurrence, age, sex, and surgical margins were not[5]. However, the results of Pierie et al[9] and Clary et al[10] showed that surgical margin was a predictor of tumor recurrence, this needs confirmation.
Immunohistochemical studies have shown that up to 94% of patients with GISTs could express CD117 (the c-kit gene product). The c-Kit protein, a typical type-III tyrosine-kinase receptor, is encoded by the proto-oncogene c-kit[11-13], and is essential for the development of the interstitial cells of Cajal[14]. The occurrence of activating mutations involving exon II of c-kit in sporadic GISTs indicates the importance of this protein in GIST tumorigenesis. There is the same mutation gene in intestinal gastric carcinoma[15]. The first case in which imatinib mesylate (Gleevec; STI-571), an inhibitor of c-kit tyrosine kinase activity, was used successfully to treat a patient with a GIST was reported in Finland by Joensuu in 2001[16].
We retrospectively analyzed the clinical outcomes of GIST patients who did and did not receive STI-571 to determine which factors in GIST patients might predict early recurrence and to show the benefit from immediate treatment with STI-571. We hypothesized that treatment with STI-571 would be successful in treating patients with recurrent giant malignant GISTs after resection. The aim of this study was to evaluate the factors determining early recurrence, prognostic factors for giant GISTs, and the effect of imatinib on recurrent GISTs after resection.
The medical records of all patients from June 1996 through December 2003 referred to the Tri-Service General Hospital were reviewed. Inclusion criteria were that the tumor originated from the gastrointestinal tract, GISTs were diagnosed by immunochemical staining for c-kit, and the tumor size was larger than 10 cm, pathologists confirmed the latter two items. Patients with a primary tumor less than 10 cm in size (n = 47) were excluded.
Twenty-three patients (11 men, 12 women) were included in this retrospective analysis. All data for the included patients were recorded, such as basic characteristics, tumor size and location, surgical margin, recurrence, palliative treatment pattern, and survival results. The timing of applying STI-571 was at tumor recurrence after surgical resection, proved by imaging studies. A dose of 400 mg twice a day was used.
The pathologists at Tri-Service General Hospital determined the tumor grade, tumor size and the presence of a tumor-free surgical margin. A negative surgical margin was defined as removal of all gross tumor tissues at operation (n = 12) and a histological examination that confirmed the margin to be free of tumor invasion. A positive surgical margin implied that total removal of gross tumor tissues was not confirmed by histological examination. This condition was usually combined with multiple organ involvement, tumor rupture during operation, or perforation (n = 11). Local recurrence was defined as a tumor recurring within the abdominal cavity, exclusive of the liver. Metastasis was defined as tumor recurrence occurring in the liver or in extra-abdominal sites. The tumor shrinkage effect of STI-571 was defined using computed tomography scans of tumor size before and after 6 mo of treatment. Complete response was tumor disappearance, partial response was tumor shrinkage by 25-75%, stable disease was tumor unchanged in size, and disease progress was tumor enlargement by more than 150%.
When the interval to tumor recurrence was calculated, the initial event was taken as the time of curative surgery and the terminal event was defined as the time of detection of the recurrent tumor at a specific site (peritoneum, liver, or extra-abdominal). For survival analysis after the onset of recurrence, the terminal event was taken as death resulting from the malignant neoplasm, or the last follow-up date for surviving patients. The survival analysis was tested by the Kaplan-Meier method, and statistical differences were tested using the Log-rank test. The numeric data were tested by Student’s t test and the category data were tested by the Fisher’s exact test. P<0.05 was considered statistically significant for all comparisons.
The mean age of the patients was 56.2±16.9 years and the mean follow-up time of patients who were alive was 29.1±25.8 mo (range 4.6-87.9 mo). The mean tumor size was 15.9±8.4 cm.
The clinical characteristics of GIST patients are shown in Table 1. Nine patients received STI-571 and 13 did not. These two groups were not different in any of the clinical characteristics. Among the patients who received STI-571 in the palliative treatment, three received repeated resection, two received trans-arterial chemo-embolization, and two received chemotherapy. Among the patients who did not receive STI-571, only two received chemotherapy.
| Total (n = 23) | STI-571 (n = 9) | No STI-571 (n = 14) | P | |
| Age (yr) | 56.2±16.9 | |||
| ≤50 yr | 40.2±11.9(5) | 39.0±10.8(4) | 0.8 | |
| >50 yr | 67.3±5.8(4) | 66.7±11.0(10) | 0.9 | |
| Sex | ||||
| Male | 11 | 2 | 9 | 0.08 |
| Female | 12 | 7 | 5 | |
| Tumor size (cm) | 15.9±8.4 | 20.7±9.9 | 12.8±5.4 | 0.06 |
| Location | ||||
| Gastroduodenal | 14 | 6 | 8 | 0.9 |
| Intestinal | 9 | 3 | 6 | |
| Surgical margin | ||||
| Negative | 12 | 4 | 8 | 0.68 |
| Positive | 11 | 5 | 6 | |
| Recurrence | 14 | 7 | 7 | |
| Local | 11 | 5 | 6 | 0.59 |
| Distal metastasis | 6 | 4 | 2 | |
| Treatment of recurrence | 14 | 7 | 7 | |
| Repeat resection | 3 | 3 | 0 | |
| TACE | 2 | 2 | 0 | |
| Chemotherapy | 3 | 2 | 2 | |
| Mean recurrence time (mo) | 15.2±11.8 | 6.9±5.7 | 0.15 |
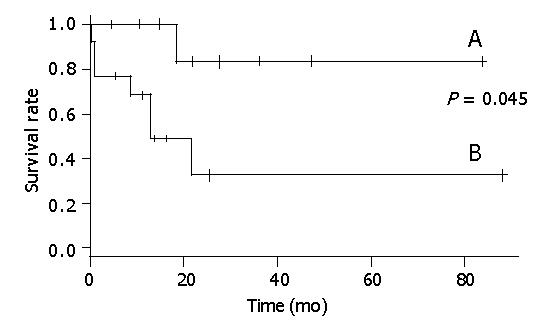
Seven patients who had tumor recurrence and did not receive STI-571, all died within 21 mo (Figure 1). Of the seven patients who had tumor recurrence but received STI-571, one died after 17.3 mo. The general characteristics of these two groups were not different.
The effects and side effects of STI-571 are shown in Table 2. The tumor shrinkage rate was 66% (6/9). Complete response occurred in two patients, partial response in four, disease progress in one, and disease re-progression after stopping STI-571 occurred in one. The side effects were skin rash, edema, gastrointestinal upset, disease progress, and abnormal liver function.
| Number (n = 9) | |
| Tumor shrinkage | 6 |
| Complete response | 2 |
| Tumor shrinkage ≥75% | 0 |
| 50% <Tumor shrinkage <75% | 3 |
| 25% <Tumor shrinkage <50% | 1 |
| Tumor shrinkage ≤25% | 2 |
| Tumor enlargement (150%) | 1 |
| Tumor re-enlargement (150%) | 1 |
| Side effect | 8 |
| Skin rash | 4 |
| Edema | 5 |
| Gastrointestinal upset | 1 |
| Disease progress | 1 |
| Abnormal liver function | 1 |
In our series, the effect of STI-571 on tumor shrinkage was obvious, the mean shrinkage of tumor size was 5.3±2.4 cm (range, 2 cm to 8 cm). Seven of nine patients who received STI-571 had tumor shrinkage, tumors completely disappeared in two patients (2/7), and partially disappeared in four (4/7). Only one patient had his tumor enlarged from 10 cm to 15 cm (1/7). The tumor in one special patient showed shrinkage after STI-571 was used for the first time, but proved resistant to re-treatment with imatinib for tumor enlargement. Two patients received STI-571 immediately after surgical resection due to their very large GISTs (25 and 30 cm). These two patients had no tumor recurrence within 14 and 4 mo of follow-up.
Table 3 shows the prognostic variables for survival analysis in GIST patients. Surgical margin and use of STI-571 were significant prognostic variables (log-rank test, P<0.05), while age, sex, and tumor location were not significant prognostic variables.
| Variable | Mortality (n = 8) | Log-rank test |
| Age (yr) | 0.085 | |
| ≤50 yr | 1/9 | |
| >50 yr | 7/14 | |
| Sex | ||
| Male | 4/11 | 0.95 |
| Female | 4/12 | |
| Location | 0.872 | |
| Gastroduodenal | 5/14 | |
| Intestinal | 3/9 | |
| Surgical margin | 0.047 | |
| Negative | 2/12 | |
| Positive | 6/11 | |
| STI-571 | 0.045 | |
| Yes | 1/9 |
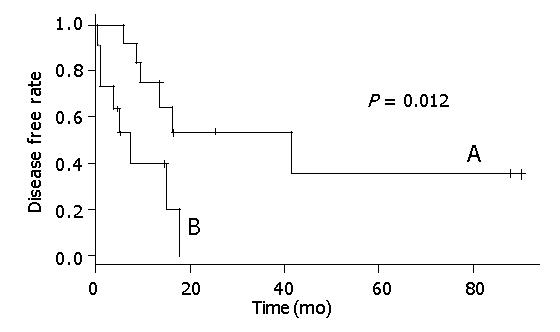
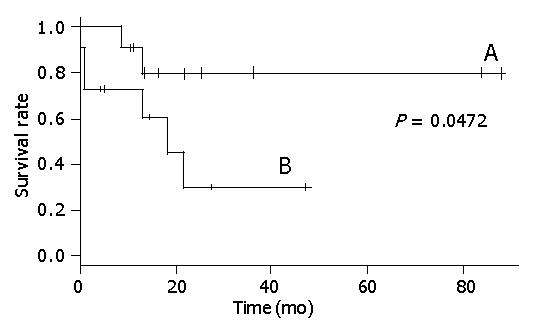
Figure 2 shows that there was a statistical relationship between surgical margin and disease-free rate. When the surgical margin was negative, the 5-year disease-free rate was nearly 40%. Conversely, when the surgical margin was positive, the two-year disease-free rate was 0%. The relation between surgical margin and survival rate is shown in Figure 3. The 5-year survival rate of patients with a negative surgical margin was 80% and the 2-year survival rate of patients with a positive surgical margin was 28%. The effect of STI-571 is shown in Figure 1. The 5-year survival rate of patients who received STI-571 was 80%, and that of patients who did not receive STI-571 was 30%. The effect of STI-571 in prolonging life span was significant.
In this series, we retrospectively analyzed 23 patients with giant GISTs (tumor size >10 cm) and found that surgical margin was an independent factor for tumor recurrence (Figure 2). A negative surgical margin meant total tumor removal by surgical resection. This is similar to previous studies[9,10]. This result indicated that the factors that could cause a positive surgical margin were the risk factors for tumor recurrence, such as tumor rupture, multiple organ involvement, and margin involvement on histological examination.
The 5-year survival rate was significantly better in patients with a negative surgical margin (Figure 3). Tumor rupture before or during resection is another predictor of poor outcome[6]. Lewis also showed that gross margin-free tumor resection gave a good prognosis[17].
In our series, good results were obtained for patients who received STI-571, and the survival rate of patients who received STI-571 was significantly higher than that of the patients who did not receive STI-571. A European Organization for Research and Treatment of Cancer (EORTC) in a soft tissue and bone sarcoma group phase II study[18] showed that STI-571 was well tolerated at a dose of 400 mg twice a day. Response rate in the EORTC study for GISTs was 7% complete response, 25% partial response, 24% stable disease and 14% disease progression, respectively. Tumor resistance could have been primary or acquired after several months of drug administration, but the mechanism is unknown[19]. The most common side effects were anemia (92%), periorbital edema (84%), skin rashes (69%), and fatigue (76%)[18]. This was consistent with our results.
A tumor size greater than 10 cm is the worst prognostic factor for malignant GISTs[20,21]. In a study of 100 patients with GISTs, multivariate analysis showed that tumor size was an independent prognostic factor for survival. Patients with giant GISTs (>10 cm) had a disease-specific 5-year survival of only 20% after resection[5]. Although our study was retrospective, treatment in the STI-571 and non-STI-571 eras was also different. The average size of the STI-571 group (20.7 cm) was larger than that of the non-STI-571 group (12.8 cm), although the difference was not significant. All included patients had a tumor diameter greater than 10 cm. In patients not given STI-571, the 5-year survival rate was 30%, similar to the previous study. The 5-year survival rate of patients given STI-571 was nearly 80%, and STI-571 treatment could prolong the survival time even with tumor recurrence after surgical resection.
In our series, two patients had tumor recurrences within 15 and 9 mo after resection and received only systemic chemotherapy. These two patients died within six months of chemotherapy. In contrast, the patients with giant GISTs who received STI-571 obtained good results. We conclude that patients who need palliative therapy should be treated in combination with STI-571 to give a better prognosis.
Surgical treatment is still essential, even for the largest GISTs[4], and complete surgical resection remains the only curative treatment for malignant GISTs[22]. However, significant risks of recurrence remain particularly in those tumors that demonstrate necrosis, high mitotic activity, and large size[22]. The diagnostic accuracy of c-kit staining, careful surgical planning to achieve a complete resection, and the availability of imatinib to treat metastatic diseases contribute to the high five-year overall survival rate (76%)[22]. We believe that aggressive complete resection, avoidance of tumor rupture and spillage, and en-bloc resection of other involved organs remain a good policy for treating malignant giant GISTs[21,23,24].
In conclusion, surgery is still the best primary treatment for patients with GIST tumors[21]. We suggest that immediate use of STI-571 when the surgical margin is positive would improve the prognosis of patients with giant GISTs.
Assistant Editor Guo SY Edited by Wang XL and Ma JY
| 1. | Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259-1269. [PubMed] |
| 2. | Miettinen M, Virolainen M. Gastrointestinal stromal tumors--value of CD34 antigen in their identification and separation from true leiomyomas and schwannomas. Am J Surg Pathol. 1995;19:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 292] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 3. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3114] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 4. | Miettinen M, El-Rifai W, H L Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 408] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 5. | DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1797] [Cited by in RCA: 1682] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 6. | Ng EH, Pollock RE, Munsell MF, Atkinson EN, Romsdahl MM. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg. 1992;215:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 286] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Emory TS, Sobin LH, Lukes L, Lee DH, O'Leary TJ. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol. 1999;23:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 409] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 8. | Ng EH, Pollock RE, Romsdahl MM. Prognostic implications of patterns of failure for gastrointestinal leiomyosarcomas. Cancer. 1992;69:1334-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Pierie JP, Choudry U, Muzikansky A, Yeap BY, Souba WW, Ott MJ. The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch Surg. 2001;136:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 208] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Clary BM, DeMatteo RP, Lewis JJ, Leung D, Brennan MF. Gastrointestinal stromal tumors and leiomyosarcoma of the abdomen and retroperitoneum: a clinical comparison. Ann Surg Oncol. 2001;8:290-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Yarden Y, Kuang WJ, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ, Chen E, Schlessinger J, Francke U, Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6:3341-3351. [PubMed] |
| 12. | Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 926] [Cited by in RCA: 932] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 13. | Geissler EN, Ryan MA, Housman DE. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 887] [Cited by in RCA: 905] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 14. | Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K, Nishikawa S. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369-375. [PubMed] |
| 15. | Liu YQ, Zhao H, Ning T, Ke Y, Li JY. Expression of 1A6 gene and its correlation with intestinal gastric carcinoma. World J Gastroenterol. 2003;9:238-241. [PubMed] |
| 16. | Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 1324] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 17. | Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 652] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 18. | Verweij J, van Oosterom A, Blay JY, Judson I, Rodenhuis S, van der Graaf W, Radford J, Le Cesne A, Hogendoorn PC, di Paola ED. Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer. 2003;39:2006-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 274] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 19. | Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002;33:466-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 428] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 20. | Bilimoria MM, Holtz DJ, Mirza NQ, Feig BW, Pisters PW, Patel S, Pollock RE, Benjamin RS, Papadopoulos NE, Plager C. Tumor volume as a prognostic factor for sarcomatosis. Cancer. 2002;94:2441-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Mudan SS, Conlon KC, Woodruff JM, Lewis JJ, Brennan MF. Salvage surgery for patients with recurrent gastrointestinal sarcoma: prognostic factors to guide patient selection. Cancer. 2000;88:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Wu PC, Langerman A, Ryan CW, Hart J, Swiger S, Posner MC. Surgical treatment of gastrointestinal stromal tumors in the imatinib (STI-571) era. Surgery. 2003;134:656-665; discussion 665-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Crosby JA, Catton CN, Davis A, Couture J, O'Sullivan B, Kandel R, Swallow CJ. Malignant gastrointestinal stromal tumors of the small intestine: a review of 50 cases from a prospective database. Ann Surg Oncol. 2001;8:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 24. | Pidhorecky I, Cheney RT, Kraybill WG, Gibbs JF. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol. 2000;7:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 316] [Article Influence: 12.6] [Reference Citation Analysis (1)] |