INTRODUCTION
In China, the incidence of HCC is very high[1], and surgical operation, chemotherapy, and interventional therapy are the common therapies. But only a few patients of early stage without extrahepatic malignancy are indicated for operation, and for the advanced cases, chemotherapy and interventional therapy usually can not achieve a satisfactory effect. Therefore, it is urgent to find a novel strategy to prevent the proliferation of malignant cells. It is a hot spot at present to study immunogene therapy.
CD40 ligand (CD40L) is a 39 kD glycoprotein which is expressed on lymphocytes and non-lymphocytic leukocytes[2]. It is a member of the tumor necrosis factor family, and binds to CD40 on the membrane of antigen-presenting cells (APC)[3,4]. CD40-CD40L interaction plays a crucial role in the activation of APC and the initiation of both humoral and cellular immune responses[5-7].
Thus, gene transfer of CD40L has been proposed as an efficient means to treat malignancies[8]. CD40L may be used as a target molecule for immunotherapy or prophylaxis against HCC. For this purpose, we constructed the recombinant mCD40L eukaryotic expression vector, and expected that it would provide experimental data for the treatment of HCC.
MATERIALS AND METHODS
Cell lines and culture condition
Mouse splenocytes were obtained from female BALB/c mice. Cells were maintained in RPMI 1640 medium, supplemented with 10 mL/L FCS and 1 mmol/L glutamine, activated with Con A for 5 h. H22 cell line was provided by the Department of Biochemistry, Fourth Military Medical University, Xi’an, China. Cells were maintained in RPMI 1640 medium, supplemented with 10 mL/L FCS, 1 mmol/L glutamine, and 100 kU/L penicillin.
Plasmids and strain
The eukaryotic expression vector pcDNA3.1+ was presented by Dr. Chun Deng, Michigan College, USA. The T vector pUCm-T was provided by the Bioasia, Shanghai. E.coli strain JM109 was purchased from Invitrogen Life Technologies (Burlington, ON, Canada).
Construction of recombinant mCD40L eukaryotic expression vector
A cDNA fragment coding for the full open reading frame of mouse CD40L gene was cloned by reverse transcription polymerase chain reaction (RT-PCR) from Con A stimulated mouse spleen cells using Taq polymerase. Two primers specific for the mouse CD40L gene were used, namely, the sense primer (5’-GACGCTAGCATGATAGAAACATACAGCCAACCT-3’) and the antisense primer (5’-GCCGAATTCTCAGAGTTTGAGTAAGCCAAAAGA-3’). PCR conditions included 1 cycle at 94 °C for 5 min for pre-denaturation; 35 amplification cycles each consisting of denaturation at 94 °C for 60 s, annealing at 60 °C for 50 s, and extension at 72 °C for 90 s; followed by a further extension at 72 °C for 10 min. The cloned cDNA fragment was ligated into the pUCm-T vector to form pUCm-T-CD40L. The cDNA fragment of mCD40L from pUCm-T-mCD40L vector was further subcloned into the pcDNA3.1+ to form eukaryotic expression vector pcDNA3.1+-mCD40L. The recombinant expression vector pcDNA3.1+-mCD40L was amplified in E.coli JM109 and then extracted by Wizard® Plus SV Minipreps DNA Purification System (Promega, USA) according to the manufacturer’s instructions.
Identification of recombinant mCD40L eukaryotic expression vector
The full-length mCD40L-cDNA was retrieved from the gel. pcDNA3.1+-mCD40L was digested by Nhe I and EcoR I according to the afore-mentioned methods. The digested products were evaluated with 1.0% agarose gel electrophoresis. The full-length mCD40L-cDNA was successfully inserted into pcDNA3.1+.
Nucleotide sequence analysis
The correct plasmids of recombinant mCD40L eukaryotic expression vector identified by the restriction analysis were sequenced using fluorescent dideoxynucleotides (Bioasia, Shanghai, China) on an automated DNA sequencer (ABI Prism 377). The sequences were compared with the published cDNA sequence[4].
Transfection of H22 cells
H22 cells were transfected with pcDNA3.1+-mCD40L by liposome, LipofectamineTM 2000 (Gibco, USA). All the procedures were performed according to the guidance of the reagent. After 24-h exposure, cells were washed three times with medium and cultured in normal medium. Twenty-four hours after medium exchange, cells were selected in medium containing 800 μg/mL G418 (Gibco, USA). After 18 d of selection, G418-resistant clones were selected randomly from the surviving colonies. H22 cells were also transfected with pcDNA3.1+ as control group, and H22 cells that were not transfected also served as control.
Detection of mCD40L expression by RT-PCR
To demonstrate the expression of mCD40L mediated by pcDNA3.1+-mCD40L vector, total cellular RNA was extracted from the H22 cells. Synthesis and amplification of mCD40L cDNA were performed using RT-PCR. The cDNA was amplified with primers specific for mCD40L and the synthesis conditions according to the afore-mentioned methods.
Detection of mCD40L expression by immunofluorescence
The transfected cells were stabilized with 10 g/L formaldehydum polymerisatum for 30 min at 4 °C, and blocked with 100 g/L bovine serum albumin (BSA) for 1 h at room temperature, then coated with rabbit anti-mouse CD40L antibody (Dako, Japan) for 45 min at 37 °C, followed by goat anti-rabbit IgG-FITC (Boster, Wuhan) for 30 min at 37 °C. The antibody-coated cells were observed under a fluorescence microscope (Optiphot XIF, Nicon, Japan) within 24 h.
RESULTS
Evaluation of pcDNA3.1+-mCD40L
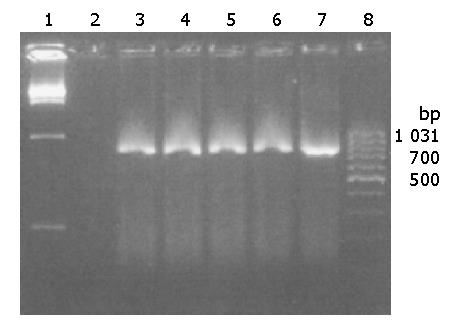
To demonstrate whether the full-length mouse CD40L-cDNA was inserted into the multi-clone sites of pcDNA3.1+ correctly, pcDNA3.1+-mCD40L was digested by Nhe I and EcoR I and evaluated with electrophoresis. Figure 1 shows that mCD40L-cDNA was successfully inserted into pcDNA3.1+.
Figure 1 Electrophoresis of recombinant plasmid pcDNA3.
1+-mCD40L. Lanes 1 and 5: DNA molecular weight marker; lane 2: The RT-PCR product of mCD40L; lane 3: pcDNA3.1+-mCD40L digested with NheI and EcoRI, pcDNA3.1+ in the upper, full-length mCD40L-cDNA in the lower; and lane 4: pcDNA3.1+-mCD40L not digested with NheI and EcoRI.
Recombinant plasmid pcDNA3.1+-mCD40L sequence analysis
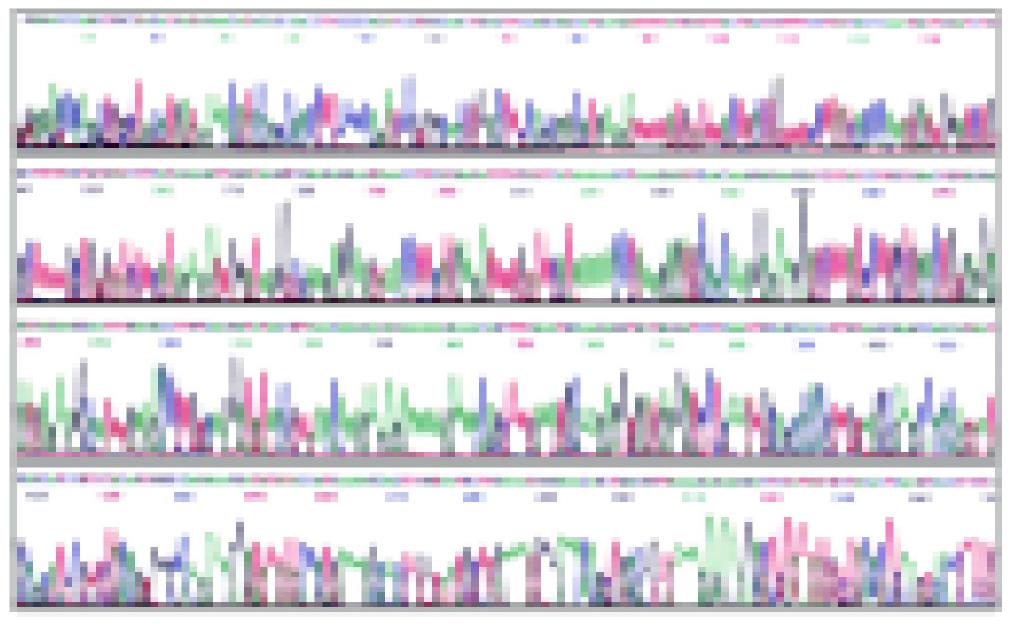
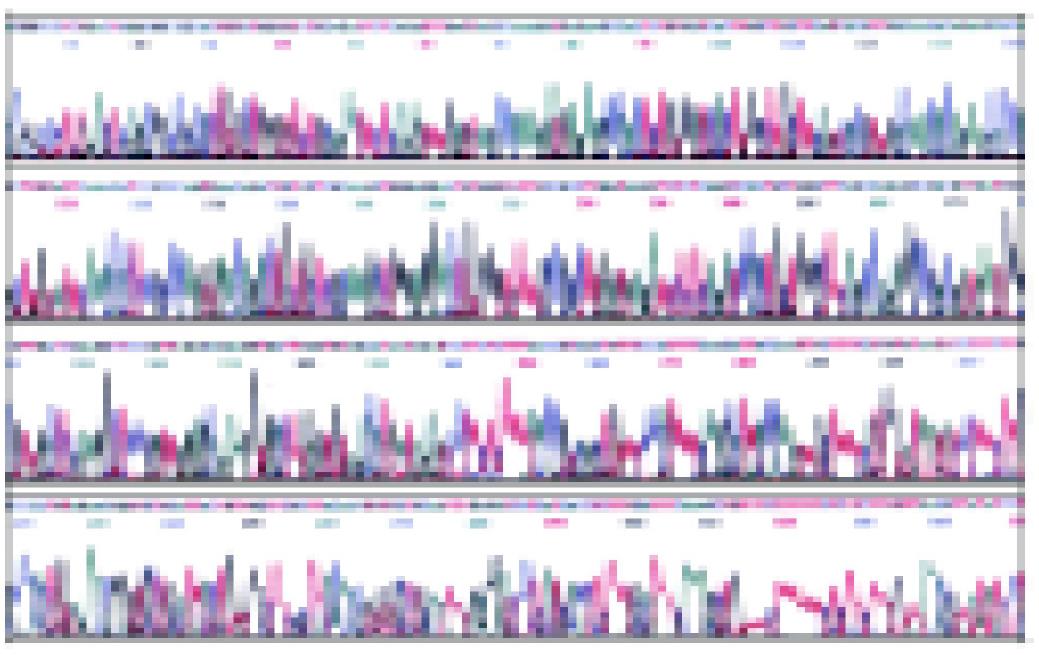
Plasmid DNA was extracted. The correct plasmids identified by restriction analysis were sequenced. The result of sequencing is shown in Figures 2, 3 and 4. It demonstrated that integral mCD40L cDNA containing start codon, stop codon and endonuclease sites was cloned into eukaryotic expression vector pcDNA3.1+.
Figure 2 The sequencing map of pcDNA3.
1+-mCD40L with M13F primer.
Figure 3 Sequencing map of pcDNA3.
1+-mCD40L with M13R primer.
Figure 4 Nucleotide splicing sequence result of pcDNA3.
1+-mCD40L with DNAstar software analysis.
RT-PCR
RT-PCR analysis showed that 0.8 kb fragments corresponding to the mCD40L cDNA were amplified with the total cellular RNA only from pcDNA3.1+-mCD40L-transduced H22 cells, but not from the cells transduced with pcDNA3.1+ (Figure 5).
Figure 5 mCD40L gene expression in H22 cells by RT-PCR.
Lanes 1 and 8: marker; Lane 2: negative control; Lanes 3-6: RT-PCR products from transfected H22 cells; Lane 7: RT-PCR product from BALB/C mouse splenocytes.
Immunofluorescence staining of transfected cells
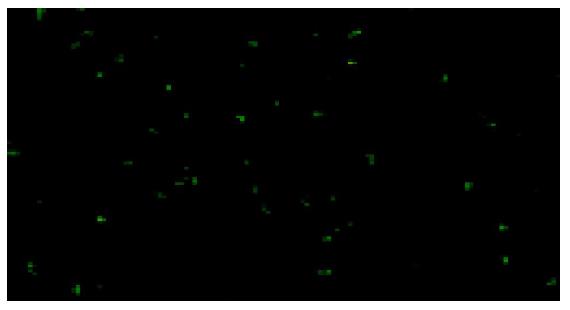
The transfected cells were coated with mCD40L antibody and fluorescent antibody. Under fluorescence microscopes, it was observed that the fluorescence staining was positive in the cytoplasm of H22 cells transfected with pcDNA3.1+-mCD40L and negative in the controls (Figure 6).
Figure 6 H22 cells transfected with pcDNA3.
1+-mCD40L. The fluorescence staining in the cytoplasm was positive. ×400.
DISCUSSION
Hepatocellular carcinoma (HCC) is a very common malignancy in China, and has a very poor prognosis[1]. Despite recent increases in therapeutic options, such as total vascular exclusion (TVE) technique used in hepatectomies of advanced and complicated hepatocellular carcinomas, combined multimodal interventional therapies which have revealed their advantages as compared with any single therapeutic regimen and play more important roles in treating unresectable HCC[9], and hepatic arterial infusion chemotherapy which may be a useful alternative for the treatment of patients with advanced HCC[10], percutaneous cryoablation which offers a safe and possibly curative treatment option for patients with HCC that can not be surgically removed[11], HCC remains one of the leading causes of death. The malignancies are often resistant to chemotherapy and metastasize to distant organs in the early stage of the disease. Even after the primary tumor is removed surgically, relapse at the primary or distant sites frequently occurs. Surgical resection itself may not accelerate cancer dissemination and increase postoperative recurrence significantly[12]. Preoperative level of circulating VEGF mRNA, especially isoform VEGF165, plays a significant role in the prediction of postoperative recurrence of HCC. Gene therapy provides new possibilities for the treatment of incurable diseases, including hepatocellular carcinoma[13].
However, the host immunity frequently fails to eliminate malignant tumors caused either by the lack of recognizable tumor antigens or by the inability of tumor antigens to stimulate an effective immune response[14,15], thus most malignant tumors evade host immune surveillance. The use of gene therapy with immunostimulatory molecules aiming at enhancing antitumoral immunity has emerged as a promising new approach to treat cancer[16,17].
CD40L-CD40 interactions on menbranes of APC, including dendritic cells (DCs), can activate APC, promote effective antigen presentation, express costimulatory and adhesion molecules, and up-regulate cytokine and chemokine production. For instance, CD40L-CD40 interactions strongly promote Th1 differentiation and provide a strong signal for interleukin (IL)-12 production, even in the presence of Th2 cytokines[18]. The endogenous production of IL-12 resulting from CD40L-CD40 interaction may play a role in the persistence of antitumor effects[19]. CD40 activation through CD40L-CD40 interaction may be used as a potential treatment tool of malignant neoplasms[20]. The rational for transducing tumor cells with CD40L is to convert these cells into stimulators of APC, an effect leading to enhanced presentation of tumor antigens to T cells and activation of antitumor immune responses. In fact, CD40L-CD40 interactions have been demonstrated to overcome tumor-specific CD4+ and CD8+ tolerance and induce antitumor immunity[21,22]. It remains possible that combining CD40L gene with another immunotherapeutic modality (e.g., IL-12, CD80) or with a tumor associated antigen (e.g., alpha fetoprotein, AFP) gene therapy would be still better. Using mCD40L gene or combining with IL-12 gene transduced leukemia cell therapy for chronic lymphocytic leukemia (CLL)[23], primary B-CLL[24,25] and acute leukemia[26] has provided encouraging results. Treatment not only appears capable of inducing a cellular anti-leukemia immunity, but also may have a direct effect on leukemia cells by inducing latent sensitivity to Fas (CD95)-dependent leukemia cell apoptosis[26]. Recently, many investigators have reported that ex vivo transduction of tumor cells with the mCD40L gene or in vivo transfer of the mCD40L gene is able to induce antitumor immunity against different tumor cell lines in subcutaneous tumor models, such as lung cancer[27,28], colon cancer[29,30], urologic malignancies[31-33], melanoma[34] and malignant mesothelioma[35]. In HCC, Schmitz et al[8] used adenovirus-mediated CD40 ligand gene therapy for orthotopic hepatocellular carcinoma, and demonstrated that it abrogated HCC cell tumorigenicity when expressing mCD40L gene, and led to complete tumor eradication and long-term survival in most of the treated animals. Yanagi et al[36] showed that Flt3L and CD40L significantly induced antitumor immunity against MH134 cells presumably through both innate and adaptive immunity. These data reveal that administration of mCD40L gene might provide an efficient and safe treatment for HCC.
In summary, mCD40L gene has shown its profound effect on HCC treatment in animal models. In our experiment, we could successfully construct the recombined mCD40L eukaryotic expression vector, and the mCD40L expressed in H22 cells effectively. This, we believe, provides experimental data for gene therapy and immunotherapy of HCC.