Published online May 21, 2005. doi: 10.3748/wjg.v11.i19.2994
Revised: March 2, 2004
Accepted: April 16, 2004
Published online: May 21, 2005
AIM: To investigate the protective effect and possible mechanism of L-arginine preconditioning on ischemia and reperfusion injury associated with small bowel transplantation (SBT).
METHODS: Male inbred Wistar rats weighting between 180 and 250 g were used as donors and recipients in the study. Heterotopic rat SBT was performed according to the techniques of Li and Wu. During the experiment, intestinal grafts were preserved in 4 °C Ringer’s solution for 8 h before being transplanted. Animals were divided into three groups. In group 1, donors received intravenous L-arginine (50 mg/kg, 1 mL) injection 90 min before graft harvesting. However, donors in control group were given normal saline (NS) instead. In group 3, six rats were used as sham-operated control. Specimens were taken from intestinal grafts 15 min after reperfusion. Histological grading, tissue malondialdehyde (MDA) and myeloperoxidase (MPO) levels were assessed. The graft survival of each group was monitored daily until 14 d after transplantation.
RESULTS: Levels of MDA and MPO in intestine of sham-operated rats were 2.0±0.22 mmol/g and 0.66±0.105 U/g. Eight hours of cold preservation followed by 15 min of reperfusion resulted in significant increases in tissue MDA and MPO levels. Pretreatment with L-arginine before graft harvesting resulted in lower enhancement of tissue levels of MDA and MPO and the differences were significant (4.71±1.02 mmol/g vs 8.02±3.49 mmol/g, 1.03±0.095 U/g vs 1.53±0.068 U/g, P<0.05). Besides, animals in L-arginine pretreated group had better histological structures and higher 2-wk graft survival rates comparing with that in NS treated group (3.3±0.52 vs 6±0.1, 0/6 vs 6/6, P<0.05 or 0.01).
CONCLUSION: L-arginine preconditioning attenuates ischemia and reperfusion injury in the rat SBT model, which was due to antioxidant activities partially.
- Citation: Cao B, Li N, Wang Y, Li JS. Protective effect of L-arginine preconditioning on ischemia and reperfusion injury associated with rat small bowel transplantation. World J Gastroenterol 2005; 11(19): 2994-2997
- URL: https://www.wjgnet.com/1007-9327/full/v11/i19/2994.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i19.2994
Small bowel transplantation (SBT) has become an accepted therapy for intestinal disease in patients who require total parenteral nutrition. Although the results of SBT have dramatically improved during the past few years, ischemia-reperfusion injury (IRI), rejection and infection continues to be a major obstacle.
Ischemic preconditioning is the process in which one or more brief periods of ischemia with intermittent reperfusion confer a state of protection against subsequent long-term IRI. This phenomenon has been reported in small intestine[1]. During the study an increase in NO synthesis was detected after intestinal preconditioning, and the protection induced by this process could be partly inhibited by the addition of the NO synthesis inhibitor L-NAME, so the preconditioning response might depend on the release of endogenous protective substances such as NO. Further investigation confirmed the protective effect of ischemic preconditioning on cold preservation and reperfusion injury in SBT[2].
L-arginine is the substrate of NO in vivo. Studies showed L-arginine application could improve graft morphology, mucosal structure and mucosal barrier function after SBT[3,4]. Another report[5] confirmed sustained NO production via L-arginine administration ameliorated effects of intestinal IRI. In the studies mentioned above L-arginine was given to recipients before reperfusion, it is uncertain whether the administration of L-arginine to donor has the same effect, so the purpose of this study was to investigate the effect of L-arginine preconditioning on graft IRI.
Male inbred Wistar rats weighting between 180 and 250 g (Shanghai Laboratory Animal Center of the Chinese Academy of Sciences) were used as donors and recipients. Housed and fed at the Animal Center of Jinling Hospital, the rats were made to get accustomed to the environment for at least 7 d before experiment. The donor and recipient were paired according to similar body weight.
Rat SBT was performed according to the techniques of Li and Wu. Briefly, the total length of intestinal graft was harvested with the vessel pedicle composed of superior mesenteric artery (SMA) and portal vein (PV). The graft intestine was preserved in Ringer’s solution at 4 °C for 8 h before being transplanted heterotopically. In recipient the graft SMA and PV were anastomosed to the abdominal aorta and left renal vein respectively.
Animals were randomly assigned to the following groups. In group 1 (L-arginine), the donors were infused with L-arginine (50 mg/kg, 1 mL) 90 min before graft harvesting. In group 2 (control), donor animals received normal saline (NS), 1 mL injection instead of L-arginine. In group 3 (sham-operated), six rats were used as sham-operated control, of which the intestine was mobilized without enterectomy to exclude the influence of operation.
Tissue malondialdehyde (MDA) and myeloperoxidase (MPO) activities were determined as indexes of tissue antioxidant status and neutrophils accumulation. The samples were obtained after 15 min of reperfusion and stored at -70 °C. Tissue MDA activity was determined by thiobarbituric acid test[6]. To measure tissue MPO activity, frozen intestine was thawed and extracted for MPO, following the homogenization and sonication procedure as described by Krawisz[7]. MPO activity in supernatant was measured and calculated from the absorbance (at 460 nm) changes that resulted from decomposition of H2O2 in the presence of O-dianisidine.
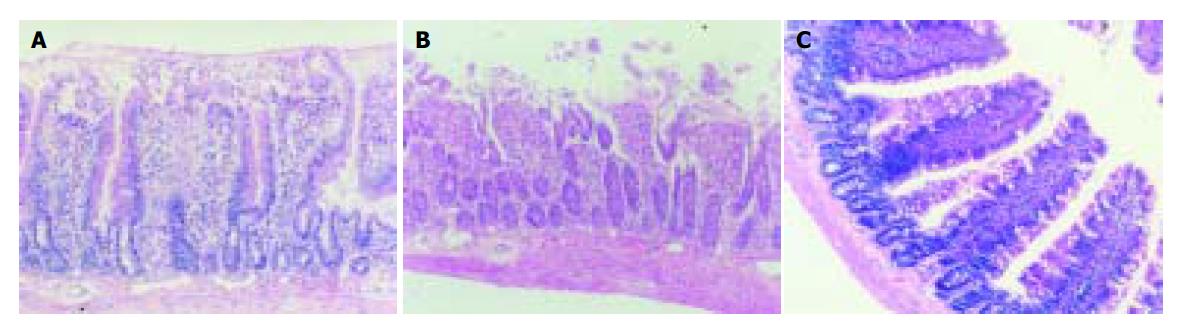
Full-thickness graft specimens for histological examination were fixed in 10% buffered formalin and processed for H&E light microscopy. The sections were graded for tissue injury using a scale of 0 (none) to 8 (severe)[8] based on the following criteria: (0) normal mucosa; (1) development of subepithelial (Gruenhagen’s) spaces at villus tips; (2) extension of the subepithelial space with moderate epithelial cell lifting from the lamina propria; (3) massive lifting down sides of villi, some denuded tips; (4) denuded villi, dilated capillaries; (5) disintegration of the lamina propria; (6) crypt layer injury; (7) transmucosal infarction. All histological analyses were performed in a blinded fashion so as to avoid bias.
After operation the graft survival of each group was monitored daily until 14 d after transplantation.
All results were expressed as mean±SD. Significance was determined using one-way analysis of variance (ANOVA) for repeated measures followed by Newman-Keuls correction where appropriate. For comparison of graft survival, Mann-Whitney U test was used. All the changes and differences were considered statistically significant when the P value was less than 0.05.
The results of tissue MDA and MPO contents are shown in Table 1. The tissue concentrations of MDA and MPO in intestine of sham-operated rats were 2.0±0.22 mmol/g and 0.66±0.105 U/g. Eight hours of cold preservation followed by 15 min of reperfusion resulted in significant increases in tissue MDA and MPO levels. But the increases of MDA and MPO activities were significantly lower in L-arginine group than in control group.
After 15 min of reperfusion, grafts in control group showed moderate to severe histological changes of IRI. A significant decrease in extent of IRI-related changes was observed in grafts of recipients treated with L-arginine 90 min before graft harvesting (Figure 1 and Table 2).
The results of this experiment demonstrated that 8 h of preservation induced severe IRI with none surviving for more than 2 wk in control group. The longest survival time of graft in control group was 36 h. On the other hand, all grafts in L-arginine-treated group survived for more than 2 wk (Table 3).
Improvements in immunosuppression and the decrease in severe acute rejection after clinical SBT have allowed us to focus on issues other than the aforementioned complications. SBT is considered not optimal (including the preservation solution, preservation temperature and the mechanical and technical aspects). There has been increasing evidence that small bowel IRI will cause postoperative complications such as initial graft dysfunction, endotoxemia, peritonitis and an increased risk for the development of acute and chronic rejection. To efficiently improve the result of SBT, further investigations into the prevention and treatment of IRI are necessary.
Although the exact mechanism of IRI is still unknown, studies have shown that the effects of IRI are mediated partially by reactive oxygen metabolites and abnormal recruitment of activated polymorphonuclear neutrophils (PMN). This complex process can be modulated by numerous factors such as luminal pancreatic proteases[9], phospholipase A2 (PLA-2)[10], nitric oxide (NO)[11], diamines[12], regulators of calcium influx[13], adenosine[14], and other processes associated with PMN activation in the gut[15].
Studies of intestinal IRI linking the local process to the resulting systemic changes have identified NO as a potential mediator. Although some studies showed NO was detrimental[16,17] to IRI, the majority of studies demonstrated NO was beneficial to IRI[18-20]. NO was considered to have a potent vasodilative action and a strong anti-platelet effect, in addition to prevention of neutrophil-endothelial cell interaction.
The substrate for NO synthesis is the amino acid, L-arginine, which combines with molecular oxygen in the presence of cofactors flavin mononucleotide and heme, a process that is catalyzed by NO synthase. Arginine is oxidized to produce an NO molecule and L-citrulline, which can be recycled through two intermediates back to L-arginine. Supplementation with L-arginine has been used in animal models to reduce the reperfusion injury to the heart or liver[21-23]. In SBT, L-arginine has been proved effective on improving mucosal structure and barrier function when infused intravenously before reperfusion[3,4].
As we all know, L-arginine can stimulate the immune function, so it may affect the immunosuppression treatment when given to the recipient. Therefore in our study we gave L-arginine to the donor before graft harvesting, hoping it could protect the graft from IRI without affecting anti-rejection treatment. The present study demonstrated that the graft survival rate significantly improved after a relatively low dose of L-arginine was given to donor animals 90 min before harvesting. A concomitant lower increase of tissue MDA and MPO activities were also observed. The change of MDA and MPO activities indicated L-arginine preconditioning alleviated IRI partially through attenuating the oxidative stress.
We did examine the NO activity in graft after 15 min of reperfusion. The result suggested IRI induced a marked increase in graft NO level compared with normal intestine, which accorded with other studies[24]. But the difference of NO activity between control and L-arginine group was not significant. The possible reasons may be as follows: At the time of graft harvesting, the vessel was irrigated with Ringer’s solution, so the concentration of L-arginine in graft might be very low. Low concentration of L-arginine together with the short half-life of NO and long period of preservation might explain the similar changes of NO level between the two groups. It strongly suggests that alternative cytoprotective mechanisms of L-arginine should be involved in the attenuation of IRI instead of direct vasodilative and anti-platelet effects of NO.
In conclusion, the result of this study demonstrates that L-arginine preconditioning attenuates ischemia and reperfusion injury in the rat SBT model, which was due to antioxidant activities partially. The exact role of NO in it needs further investigation.
Science Editor Zhu LH Language Editor Elsevier HK
| 1. | Hotter G, Closa D, Prados M, Fernández-Cruz L, Prats N, Gelpí E, Roselló-Catafau J. Intestinal preconditioning is mediated by a transient increase in nitric oxide. Biochem Biophys Res Commun. 1996;222:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 143] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Sola A, De Oca J, González R, Prats N, Roselló-Catafau J, Gelpí E, Jaurrieta E, Hotter G. Protective effect of ischemic preconditioning on cold preservation and reperfusion injury associated with rat intestinal transplantation. Ann Surg. 2001;234:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Mueller AR, Platz KP, Heckert C, Häusler M, Radke C, Neuhaus P. L-arginine application improves mucosal structure after small bowel transplantation. Transplant Proc. 1998;30:2336-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Mueller AR, Platz KP, Schirmeier A, Nüssler NC, Seehofer D, Schmitz V, Nüssler AK, Radke C, Neuhaus P. L-arginine application improves graft morphology and mucosal barrier function after small bowel transplantation. Transplant Proc. 2000;32:1275-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Ward DT, Lawson SA, Gallagher CM, Conner WC, Shea-Donohue T. Sustained nitric oxide production via l-arginine administration ameliorates effects of intestinal ischemia-reperfusion. J Surg Res. 2000;89:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271-278. [PubMed] |
| 7. | Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344-1350. [PubMed] |
| 8. | Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1420] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 9. | Montgomery A, Borgström A, Haglund U. Pancreatic proteases and intestinal mucosal injury after ischemia and reperfusion in the pig. Gastroenterology. 1992;102:216-222. [PubMed] |
| 10. | Siems WG, Grune T, Esterbauer H. 4-Hydroxynonenal formation during ischemia and reperfusion of rat small intestine. Life Sci. 1995;57:785-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Payne D, Kubes P. Nitric oxide donors reduce the rise in reperfusion-induced intestinal mucosal permeability. Am J Physiol. 1993;265:G189-G195. [PubMed] |
| 12. | Koshi S, Inoue M, Obayashi H, Miyauchi Y. Inhibition of post-ischemic reperfusion injury of the small intestine by diamine oxidase. Biochim Biophys Acta. 1991;1075:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Mustafa NA, Yandi M, Turgutalp H, Ovali E, Aydemir V, Albayrak L. Role of diltiazem in ischemia-reperfusion injury of the intestine. Eur Surg Res. 1994;26:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Schoenberg MH, Poch B, Moch D, Marzinzig M, Marzinzig E, Mattfeldt T, Gruber H, Beger HG. Effect of acadesine treatment on postischemic damage to small intestine. Am J Physiol. 1995;269:H1752-H1759. [PubMed] |
| 15. | Weixiong H, Aneman A, Nilsson U, Lundgren O. Quantification of tissue damage in the feline small intestine during ischaemia-reperfusion: the importance of free radicals. Acta Physiol Scand. 1994;150:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Ialenti A, Ianaro A, Moncada S, Di Rosa M. Modulation of acute inflammation by endogenous nitric oxide. Eur J Pharmacol. 1992;211:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 373] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 17. | Miura M, Ichinose M, Kageyama N, Tomaki M, Takahashi T, Ishikawa J, Ohuchi Y, Oyake T, Endoh N, Shirato K. Endogenous nitric oxide modifies antigen-induced microvascular leakage in sensitized guinea pig airways. J Allergy Clin Immunol. 1996;98:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Kubes P, Granger DN. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992;262:H611-H615. [PubMed] |
| 19. | Poss WB, Timmons OD, Farrukh IS, Hoidal JR, Michael JR. Inhaled nitric oxide prevents the increase in pulmonary vascular permeability caused by hydrogen peroxide. J Appl Physiol (1985). 1995;79:886-891. [PubMed] |
| 20. | Guidot DM, Repine MJ, Hybertson BM, Repine JE. Inhaled nitric oxide prevents neutrophil-mediated, oxygen radical-dependent leak in isolated rat lungs. Am J Physiol. 1995;269:L2-L5. [PubMed] |
| 21. | Li SQ, Liang LJ. Protective mechanism of L-arginine against liver ischemic-reperfusion injury in rats. Hepatobiliary Pancreat Dis Int. 2003;2:549-552. [PubMed] |
| 22. | Yagnik GP, Takahashi Y, Tsoulfas G, Reid K, Murase N, Geller DA. Blockade of the L-arginine/NO synthase pathway worsens hepatic apoptosis and liver transplant preservation injury. Hepatology. 2002;36:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Okoński P, Szram S, Banach M, Fila M, Bielasik K, Mussur M, Zasłonka J. Effect of L-arginine on overhydration and ultrastructure preservation of rat's heart exposed to cold cardioplegic ischaemia. Ann Transplant. 2003;8:57-62. [PubMed] |
| 24. | Platz KP, Mueller AR, Heckert C, Häusler M, Guckelberger O, Lobeck H, Neuhaus P. Nitric oxide production after syngeneic and allogeneic small bowel transplantation. Transplant Proc. 1998;30:2662-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |