Published online May 21, 2005. doi: 10.3748/wjg.v11.i19.2990
Revised: June 9, 2004
Accepted: August 5, 2004
Published online: May 21, 2005
AIM: To determine the radiosensitizing potential of docetaxel in human hepatocellular carcinoma SMMC-7721 cells and its mechanisms.
METHODS: SMMC-7721 cells were incubated with docetaxel at 0.125, 0.25, and 0.5 nmoL/L for 24 h and at 0.125 and 0.25 nmol/L for 48 h before irradiation. Radiation doses were given from 0 to 10 Gy. Cell survival was measured by a standard clonogenic assay after a 9-d incubation. The reactive oxygen species (ROS) and glutathione (GSH) are detected after being given the same dose of docetaxel for the same time.
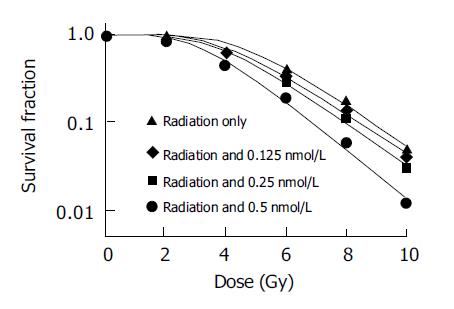
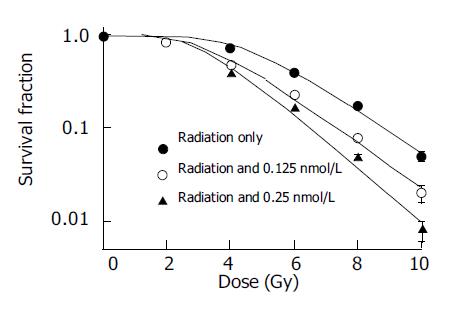
RESULTS: The sensitization enhancement ratios (SER) for SMMC-7721 cells determined at the 50% survival level were 1.15, 1.21 and 1.49 at 0.125, 0.25, and 0.5 nmol/L for pre-incubation of 24 h, respectively; the SER were 1.42, 1.67 at 0.125 and 0.25 nmol/L, for pre-incubation of 48 h, respectively. The ROS of SMMC-7721 cells increased and GSH decreased after pretreatment with the same doses of docetaxel for 24 or 48 h.
CONCLUSION: A radiosensitizing effect of docetaxel could be demonstrated unambiguously in this cell line used. In addition, our data showed that the mechanism of radiopotentiation by docetaxel probably does not involve a G2/M block in SMMC-7721 cells, and ROS generation and GSH deletion may play a key role in the radiosensitizing effect of docetaxel.
- Citation: Geng CX, Zeng ZC, Wang JY, Xuan SY, Lin CM. Docetaxel shows radiosensitization in human hepatocellular carcinoma cells. World J Gastroenterol 2005; 11(19): 2990-2993
- URL: https://www.wjgnet.com/1007-9327/full/v11/i19/2990.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i19.2990
Human hepatocellular carcinoma (HCC) is one of the major causes of death worldwide, and surgical resection is the only curative therapy, the resection rate is only 10-30%, the remaining patients are subjected to various forms of non-surgical therapy. Searching for effective chemotherapeutic agents is important to improve the survival rate of patients with advanced or recurrent HCC after surgical treatment. HCC is well known for its expression of multidrug resistance gene and its poor response to chemotherapeutic agents, thus is usually insensitive to chemotherapeutic drugs currently used in clinical setting and there is thus an urgent need for the evaluation of new options against HCC. External-beam radiation is one of the options employed currently in clinical practice. It has been recommended that a dosage greater than 50 Gy be used to kill HCC cells, however, this dose is associated with radiation-induced hepatitis and liver failure when the whole liver is treated, therefore, if radiation is used, the delivered dose should be limited to 30-35 Gy over 3-4 wk[1,2].
Docetaxel is a new toxoid structurally similar to paclitaxel, a semisynthetic product of a renewable resource, the needles of the European yew, Taxus baccata L. In comparison to paclitaxel, docetaxel is more potent as an inhibitor of microtubule depolymerization, and a less efflux of docetaxel from tumor cells was observed. Docetaxel has been assessed in various cancers, and has shown an active effect against lung cancers, breast carcinoma, the carcinoma of the head and neck[3-5].
In vitro studies provided ample evidence that docetaxel can enhance radiation sensitivity of tumor cells; treatment regimens consisting of docetaxel combined with radiotherapy have undergone extensive preclinical investigation. The focus was primarily on cell radiosensitization because docetaxel arrests cells in the radiosensitive G2/M phase of the cell cycle. In contrast with paclitaxel, docetaxel seems to be a better radiosensitizer than paclitaxel.
Studies have shown that combination therapy of docetaxel with radiation has higher efficacy and reduced effects in patients with lung cancer, head and neck cancer[6,7].
Docetaxel and agent combination with radiotherapy was also feasible and, a high complete response rate could be observed. But the related information about HCC is hardly reported and needs investigation, and the mechanism of docetaxel underlying its radioenhancing effect remains to be elucidated further. This study is to investigate the radiosensitizing effect of docetaxel and its mechanism in SMMC-7721 human HCC cells in vitro and to provide the basis for clinical application study in patients with HCC.
Docetaxel (Rhone-Poulenc Rorer Pharmaceuticals Inc.) was stored as 100 µmol/L stock solution in absolute ethanol at -80 °C. This solution was further diluted in the medium and used in the cell culture immediately before each experiment.
Human HCC SMMC-7721 cell line was obtained from Liver Cancer Institute of Fudan University. The cells were cultured in flasks with RPMI1640 (Gibco BRL, Grand Island, NY) medium supplemented with 100 IU/mL penicillin, 100 µg/mL streptomycin and 10% new-born bovine serum (Hyclone, Logan, UT) and maintained at 37 °C in a humidified atmosphere containing 50 mL/L CO2.
Exponentially growing SMMC-7721 cells were trypsinized and seeded on the basis of different density in a 60-mm culture plate (Corning, NY) with 4 mL of medium, and allowed to bind. Twenty-four hours later, the medium was discarded and replaced by different concentrations of docetaxel containing medium. The cells were incubated for 24 and 48 h and then irradiated at room temperature with an Oris 137Cesium source at doses ranging from 0 to 10 Gy. For 24 h group, docetaxel was added at 0.125, 0.25, 0.5 nmol/L and for 48 h group docetaxel was added at 0.125 and 0.25 nmol/L, respectively. For each radiation dose, four dishes were irradiated control and drug-treated cells. After irradiation, the docetaxel containing medium was replaced with equal volume of fresh medium without drug. The dishes were incubated at 37 °C in air and 50 mL/L CO2 for 9 d. The cells were fixed in ethanol, and stained with Giemsa, and manually counted. Colonies of >50 cells were considered as survivors. For all of the data obtained by cologenic assays, drug toxicity was adjusted for the surviving fraction of drug-treated cells to yield corrected survivals of 100% for non-irradiated but doctaxel-treated cells. The effect shown is therefore the sensitizing action, after the subtraction of the direct cytotoxic effect of docetaxel.
After logarithmic growth phase SMMC-7721 cells were treated with 0.125, 0.25, and 0.5 nmol/L docetaxel for 24 h, 0.125 and 0.25 nmol/L docetaxel for 48 h, the cells were incubated with 2’,7’-dichlorofluorescin diacetate (DCF/DA; 5 µmol/L; Sigma) at 37 °C for 50 min to estimate the reactive oxygen species (ROS) level. The cells were harvested and detected immediately for fluorescence intensity detection on Becton Dickinson FACSCalibur flow cytometer and data were acquired and analyzed using FACS/CELLQuest software (San Jose, CA) on a Power Macintosh 7 600/120 computer (Apple Computers, Cupertino, CA)[8], three independent experiments were repeated in each drug treated group and the control group was not given docetaxel and incubated with DCF/DA. All experiments were conducted in triplicate.
The determination of intracellular glutathione (GSH) content was conducted according to Shen et al[9], with modification. Briefly after logarithmic growth phase SMMC-7721 cells (≥106) were treated with different doses of docetaxel (0.25, 0.5, 10 nmol/L) for 24 h, harvested by trypsination, washed, resuspended in PBS and counted under phase contrast microscope. The cells suspension was centrifuged at 300 r/min for 5 min, the cell pellets were added with 0.75 mL distilled water and 0.25 mL thiosalicylic acid to precipitate protein. After centrifugation at 12000 g for 5 min at 4 °C, the supernatant was used for GSH assay. The control group was not given docetaxel. All experiments were conducted in triplicate.
The intracellular GSH content was expressed as mean±SD. Their differences between drug treated groups and the control group were analyzed with Student’s t-test.
Different doses of docetaxel incubated with SMMC-7721 cells both for 24 and 48 h show radiopotentiating effect: SERs for SMMC-7721 cells determined at the 50% survival level were 1.15, 1.21, 1.49 at 0.125, 0.25, 0.5 nmol/L for pre-incubation 24 h, respectively; SERs were 1.42, 1.67 at 0.125 and 0.25 nmol/L, for pre-incubation of 48 h, respectively. The surviving fraction of drug-treated cells was adjusted drug toxicity to yield corrected survivals of 100% for non-irradiated but doctaxel-treated cells. The effect shown is therefore the sensitizing action, after the subtraction of the direct cytotoxic effect of docetaxel (Figures 1 and 2).
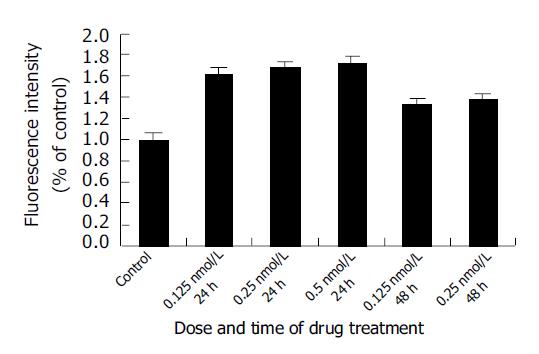
As demonstrated in Figure 3, The SMMC-7721 cells ROS level (corresponding to the fluorescence intensity) increased significantly after treatment with 0.125, 0.25, and 0.5 nmol/L docetaxel for 24 h and 0.125 and 0.25 nmol/L docetaxel for 48 h. In comparison with that of the control group, the ROS level increased by 1.61, 1.69, 1.72, 1.33 and 1.38 times, respectively.
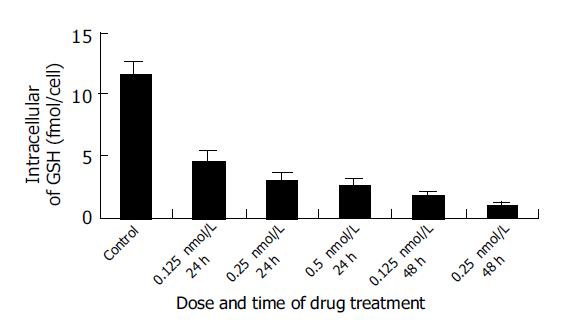
Intracellular GSH decreased significantly after treatment with 0.125, 0.25, and 0.5 nmol/L docetaxel for 24 h and 0.125 and 0.25 nmol/L docetaxel for 48 h, in comparison with that of the control group (P<0.05) (Figure 4), and on a dose- and time-dependent basis.
Docetaxel is a new toxoid cytotoxic agent which promotes tubulin assembly into microtubules and inhibits their depolymerization, in comparison to paclitaxel, docetaxel is a more active tubulin assembly promoter and microtubule stabilizer.
It has been demonstrated that docetaxel can enhance radiation sensitivity of tumor cells, potentiate tumor response, and increase the therapeutic ratio of radiotherapy, compared with tumor radioresponse, normal tissue radioresponse was much less affected by docetaxel[10]. In contrast to previously published results with paclitaxel, docetaxel seems to be a better radiosensitizer than paclitaxel.
Our results demonstrated that several doses of docetaxel incubated with SMMC-7721 cells for both 24 and 48 h shows radiosensitizing effect on this cell line, and on dose- and time-dependent basis. It is suggested that radiosensitization by paclitaxel require progression of cells through the cycle and accumulation in G2/M phase. But the colony forming assay and flow cytometric analysis of SMMC-7721 cells after treatment with different concentrations of docetaxel show that docetaxel at radiopotentiating doses did not cause any cell cycle redistribution, and the G2/M phase arrest gets apparent at a much higher dose (10 nmol/L), but the survival fraction decreases to 0 (data not shown here) at this dose of docetaxel, and this high dose docetaxel cannot be applied to radiation sensitizing study any further. So we speculate that the radiopotentiating effect of docetaxel on SMMC-7721 cells probably does not involve a G2/M arrest, and the mechanism underlying this phenomenon remains to be investigated. This is in agreement with results showing that docetaxel radiopotentiated human lung cancer cell line H460 cells, but did not cause any cell cycle redistribution and G2/M phase block. It is reported that reoxygenation of radioresistant hypoxic cells could account for radioenhancement by taxanes. Whether reoxygenation of SMMC-7721 cells by docetaxel occurred and is involved in radioenhancement needs investigation.
In order to investigate the mechanisms underlying the radioenhancement of docetaxel in SMMC-7721 cells, after SMMC-7721 cells were treated with different doses of docetaxel for 24 or 48 h, the ROS and GSH levels were determined. Our results demonstrated that the drug-treated SMMC-7721 cells ROS increased and GSH decreased significantly compared with that of the control group (P<0.05), suggesting that intracellular oxidative stress and GSH deletion occurred after docetaxel treatment, and increased ROS and GSH deletion may be involved in the radioenhancement of docetaxel in SMMC-7721 cells. According to radiation biology, radiation in the presence of oxygen caused generation of peroxide, and peroxide could damage DNA, prevent DNA from repairing, thus enhancing the radiosensitivity of DNA, potentiating the radiosensitizing effect. ROS has been found to regulate translocation of NF-κB as well as translocation of p53 and the p53-mediated apoptosis pathway[11,12]. Also it has been found that induction of apoptosis was accompanied by the generation of intracellular ROS prior to the activation of caspase-3. Recently it was reported that arsenic trioxide and l-S,R-buthionine sulfoximine (BSO) combination treatment enhanced HCC cells apoptosis through increased production of ROS[13], modulation of the ROS production inside and outside of cells may influence the cell survival during oxidative insults. Oxidative stress can occur in irradiated human leukemia “blasts”, and may play a direct role in radiation-induced apoptosis. Early studies have also demonstrated that the onset of apoptosis is associated with a fall of intracellular GSH in different cellular systems and GSH could significantly reduce apoptosis mediated by a monoclonal antibody directed to Fas antigen, and apoptosis could be almost prevented by GSH. GSH is an important cellular thiol which is regarded as the major determinant of the intracellular redox potential; on the other hand, apoptosis may be regulated by the redox status within the cells. Loss of GSH was shown to be tightly coupled with a number of downstream events in apoptosis, in caspase activation and events in chromatin, and GSH depletion may act as a link between oxidative stress and ceramide-mediated apoptosis[14]. Also it was found that decreased cells proliferation associated with decreased levels of intracellular GSH, and blockade of GSH synthesis enhanced ROS production and suppressed cells proliferation, cells with compromised cellular GSH were susceptible to redox imbalance-induced inhibition of proliferation[15]. But if there is GSH in the cells, the peroxide can be cleared and DNA damage can be repaired. Depleting the intracellular GSH by specific inhibitor BSO could increase cellular radiosensitivity. GSH plays a critical protective role in maintaining nuclear and mitochondrial DNA functional integrity, determining the intrinsic radiosensitivity of Hep G2 cells, the protective role of GSH may relate to modification of DNA damage dependent signaling.
It was reported that buthionine sulfoximine-mediated GSH depletion increased radiation-induced chromosome aberrations, apart from exchange aberrations, this could be due to reduction in the free-radical scavenging effect of GSH, a failure in rejoining of DNA double-strand breaks, or induction of apoptosis. Induction of endogenous GSH in living cells following low-dose gamma-ray irradiation is partially responsible for the appearance of radioresistance to a subsequent lethal dose of radiation, and may make it possible to use higher doses of radiation in radiotherapy for tumor patients. Radiation-induced apoptosis in radiation-resistant murine lymphoma cell line (LY-ar) cells was restored by pretreatment with paclitaxel, was correlated with lowered levels of intracellular GSH[16].
In summary, the present study demonstrates that docetaxel shows radiosensitizing effect on SMMC-7721 HCC cells in vitro. It leads to ROS generation and GSH deletion, subsequently results in redox imbalance in SMMC-7721 cells, and it is speculated that the redox imbalance caused by docetaxel may play an important role in the radioenhancement in SMMC-7721 cells. As far as we know, this is the first report concerning radiosensitization and of docetaxel and liver carcinoma the role of redox imbalance by docetaxel in the radiosensitization so far. Our results not only provide the basis for further in vivo and clinical studies in HCC but also contribute to understanding the pharmacology of docetaxel further.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Guo WJ, Yu EX. Evaluation of combined therapy with chemoembolization and irradiation for large hepatocellular carcinoma. Br J Radiol. 2000;73:1091-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Aguayo A, Patt YZ. Nonsurgical treatment of hepatocellular carcinoma. Semin Oncol. 2001;28:503-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Petrioli R, Pozzessere D, Messinese S, Sabatino M, Ceciarini F, Marsili S, Correale P, Fiaschi AI, Voltolini L, Gotti G. Weekly low-dose docetaxel in advanced non-small cell lung cancer previously treated with two chemotherapy regimens. Lung Cancer. 2003;39:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Coleman RE, Howell A, Eggleton SP, Maling SJ, Miles DW. Phase II study of docetaxel in patients with liver metastases from breast cancer. UK study group. Ann Oncol. 2000;11:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Glisson BS, Murphy BA, Frenette G, Khuri FR, Forastiere AA. Phase II Trial of docetaxel and cisplatin combination chemotherapy in patients with squamous cell carcinoma of the head and neck. J Clin Oncol. 2002;20:1593-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Wu HG, Bang YJ, Choi EK, Ahn YC, Kim YW, Lim TH, Suh C, Park K, Park CI. Phase I study of weekly docetaxel and cisplatin concurrent with thoracic radiotherapy in Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;52:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Pradier O, Rave-Fränk M, Lehmann J, Lücke E, Boghun O, Hess CF, Schmidberger H. Effects of docetaxel in combination with radiation on human head and neck cancer cells (ZMK-1) and cervical squamous cell carcinoma cells (CaSki ). Int J Cancer. 2001;91:840-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Lin HL, Liu TY, Chau GY, Lui WY, Chi CW. Comparison of 2-methoxyestradiol-induced, docetaxel-induced, and paclitaxel-induced apoptosis in hepatoma cells and its correlation with reactive oxygen species. Cancer. 2000;89:983-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Shen HM, Yang CF, Ong CN. Sodium selenite-induced oxidative stress and apoptosis in human hepatoma HepG2 cells. Int J Cancer. 1999;81:820-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Creane M, Seymour CB, Colucci S, Mothersill C. Radiobiological effects of docetaxel (Taxotere): a potential radiation sensitizer. Int J Radiat Biol. 1999;75:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Kheradmand F, Werner E, Tremble P, Symons M, Werb Z. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science. 1998;280:898-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 299] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 12. | Martinez JD, Pennington ME, Craven MT, Warters RL, Cress AE. Free radicals generated by ionizing radiation signal nuclear translocation of p53. Cell Growth Differ. 1997;8:941-949. [PubMed] |
| 13. | Kito M, Akao Y, Ohishi N, Yagi K, Nozawa Y. Arsenic trioxide-induced apoptosis and its enhancement by buthionine sulfoximine in hepatocellular carcinoma cell lines. Biochem Biophys Res Commun. 2002;291:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Lavrentiadou SN, Chan C, Kawcak T, Ravid T, Tsaba A, van der Vliet A, Rasooly R, Goldkorn T. Ceramide-mediated apoptosis in lung epithelial cells is regulated by glutathione. Am J Respir Cell Mol Biol. 2001;25:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Noda T, Iwakiri R, Fujimoto K, Aw TY. Induction of mild intracellular redox imbalance inhibits proliferation of CaCo-2 cells. FASEB J. 2001;15:2131-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Nguyen LN, Munshi A, Hobbs ML, Story MD, Meyn RD. Paclitaxel restores radiation-induced apoptosis in a bcl-2-expressing, radiation-resistant lymphoma cell line. Int J Radiat Oncol Biol Phys. 2001;49:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |