INTRODUCTION
Acute liver injury (ALI) is a co-operative consequence of endotoxemia, microcirculation dysfunction as well as inflammatory cells (such as macrophage, lymphocyte) that release inflammatory mediators and cytokines (such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1)) when stimulated. ALI is mostly induced by viral hepatitis, alcoholism, iron overload, or drug toxicity. It has a very high morbidity and mortality. The treatment might be anti-inflammatory or antioxidant action. Many modern Western medicines have been used to remedy ALI, but strategies are difficult to achieve satisfied outcomes due to their side effects. However, some traditional Chinese herbs (such as Radix Paeonia Pall, Radix Astragali, Radix Salviae Miltiorrhizae, Cordycep sinensis, Ginkgo biloba, Picrorhiza scrophulariflora) have been found to have particular advantages in therapeutic research of ALI and other liver disease for their definite effectiveness, cheap prices and negligible side effects[1-6]. Traditional Chinese medicine (TCM) treatment is based on overall analysis of symptoms and signs, and the physical condition of the patient[7]. Fufanghuangqiduogan (FFHQ) is an extract of prescription TCM consisting of Radix Astragali, Radix Paeonia lactiflora, etc. The present study aims at exploring the effects of FFHQ on the prevention of immunologic ALI induced by Bacillus Calmette Guerin (BCG)+ lipopolysaccharide (LPS) in mice and chemical ALI induced by CCl4 in mice, and the content of malondialdehyde (MDA) and the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-px) in mice liver homogenate were determined in order to investigate its possible mechanisms.
MATERIALS AND METHODS
Drugs and materials
CCl4, purchased from Beijing Chemical Factory, was diluted to 0.1% in vegetable oil. LPS from Escherichia coli was obtained from Sigma Chemical Co. (St. Louis, MO, USA). BCG was purchased from Institute of Shanghai Biological Products. FFHQ is an extract of traditional Chinese herbs consisting of Radix Astragali, Radix Paeonia lactiflora and Radix Glycyrrhizae purchased from Anhui Heyitang Pharmacy, China. These herbs mixed up in specified ratio (1:4:0.8) were boiled with water and extracted by alcohol: 95% alcohol in liquid of these herbs by the volume proportion of 2:1 was mixed and stored at 0-4 °C for 24 h, then the sediments were filtered and the suspension including the protein and amylum was finally heated at 90-95 °C to evaporate the remaining alcohol and to obtain yellow brown powders. FFHQ was mainly composed of the total glucosides of Paeony (TGP), the total astragalosides (TAS), the total flavonoids of astragalus (TFA), astragalus polysaccharides and so on. TGP and TAS, accounting for 59.3%, were main effective components of FFHQ. FFHQ was dissolved into 0.5% sodium carboxymethylcellulose (CMC-Na) solutions before use. Commercial kits used for determining lipid peroxidation and SOD activity were obtained from the Jiancheng Institute of Biotechnology (Nanjing, China). Other chemicals used in these experiments were of analytical grade from comm-ercial sources.
Animals
Male Kunming mice (20±2 g) were obtained from the Animal Department of Anhui Medical University. Mice were maint-ained on 12-h light/dark cycles. Animals were allowed free access to food and water. All mice were fasted for 16 h prior to blood/tissue sampling. All experiments were performed in accordance with the institutional ethical guideline.
Establishment of chemical liver injury model[
8-10]
A CCl4 0.1% vegetable oil solution was injected intraperitoneally into each animal in a dose of 10 mL/kg body weight. All the mice were anesthetized with ether, then killed by cervical dislocation 16 h after CCl4 injection and trunk blood was collected into heparinized tubes (50 U/mL) and centrifuged (1500 r/min, 10 min, 4 °C). Serum was aspirated and stored at -70 °C until assayed as described below. The liver was also removed and stored at -70 °C until use.
Establishment of immunological liver injury model[
11]
A 2.5 mg dose of BCG (viable bacilli) suspended in 0.2 mL saline was injected via the tail vein into each animal, and 10 d later, injected with 7.5 µg LPS dissolved in 0.2 mL saline. The mice were anesthetized with ether, and then killed by cervical dislocation 16 h after LPS injection. The other method adopted in the case of pretreatment studies was the same as in CCl4-induced liver injury mentioned above.
Drug treatment
In the two model experiments, the animals were equally divided into six groups randomly which included normal, model control, FFHQ groups (three different doses) and bifendate. The mice in FFHQ groups received daily doses of 60, 120 or 240 mg/kg b.w. of FFHQ using an 18-gauge stainless steel animal feeding needle for 10 d prior to LPS injection and for 7 d prior to CCl4 injection, respectively. Mice in normal and model control group in the two model experiments were fed only with the same volume of vehicle.
Measurement of serum ALT, AST, TNF-α and IL-1
Serum alanine aminotransferase (ALT) and aspartate aminot-ransferase (AST) were determined using commercial kits produced by Jiancheng Institute of Biotechnology (Nanjing, China). The activities of ALT and AST were expressed as an international unit (U/L). Serum TNF-α and IL-1 were measured using commercial kits produced by Beijing Biotechnology Co., Ltd, and their levels were expressed as nanogram per milliliter.
Measurement of MDA, SOD and GSH-px in liver homogenate
Liver was thawed, weighed and homogenized in Tris-HCl (5 mmol/L containing 2 mmol/L EDTA, pH 7.4). Homog-enates were centrifuged (1000 r/min, 10 min, 4 °C) and the supernatant was used immediately for the assays of MDA and SOD. MDA, SOD and GSH-px were determined following the instructions on the kit. In brief, MDA in liver tissue was determined by the thiobarbituric acid method. All samples were assayed in triplicates. The content of MDA was expressed as nanomole per gram liver tissue. The assay for total SOD was based on its ability to inhibit the oxidation of oxyamine by the xanthine-xanthine oxidase system. The red product (nitrite) produced by the oxidation of oxyamine had an absorbance at 550 nm. One unit (U) of SOD activity was defined as the amount that reduced the absorbance at 550 nm by 50%. All samples were assayed in triplicates. Results were expressed as unit per gram liver tissue. GSH-px was measured by the DTNB method, and its content was expressed as unit per milligram protein.
Histologic analysis
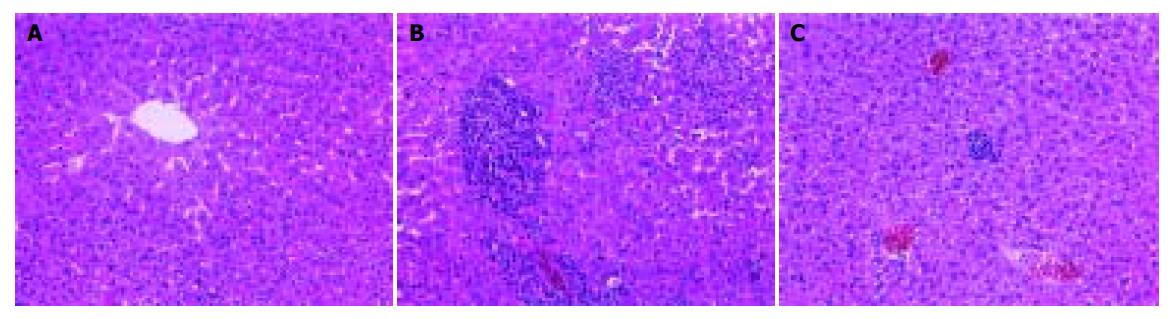
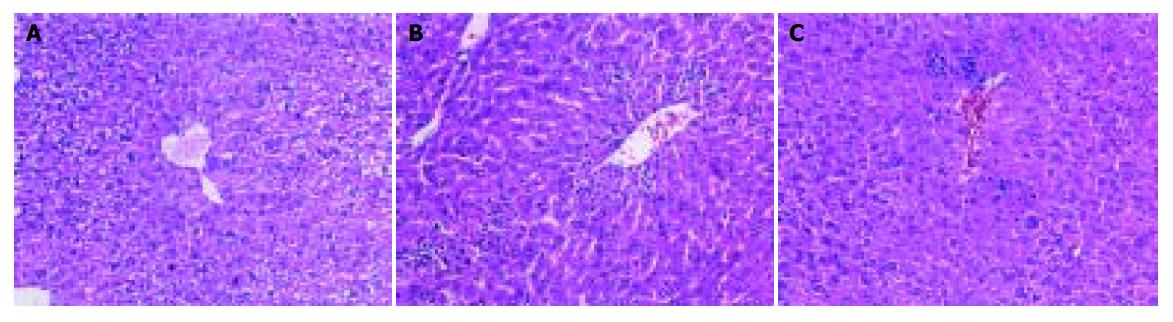
Formalin-fixed specimens were embedded in paraffin and stained with hematoxylin and eosin for conventional morphologic evaluation. After decapitation of rats, small liver specimens were placed in 100 mL/L formalin solution and processed routinely by embedding in paraffin. Tissue sections (4-5 µm) were stained with hematoxylin and eosin and examined under light microscope (Olympus, Japan). An experienced histologist who was unaware of the treatment conditions made histologic assessments.
Statistical analysis
All values were presented as mean±SD. Statistical analysis of the data for multiple comparisons was performed by one-way analysis of variance followed by Duncan’s test. For a single comparison, the significance of differences between means was determined by Student’s t-test. A level of P<0.05 was taken as statistically significant.
DISCUSSION
FFHQ was an extract of Chinese herbs prescription that has various kinds of pharmacologic actions. In the prescription, the main Chinese herbs such as Radix Astragali and Radix Paeonia lactiflora have been used to relieve the pain and be an effective prescription for treatment of liver disease and other diseases[1,2,7,12,13]. FFHQ has some active compounds, such as TGP (consist of paeoniflorin, albiflorin, benzoylpaeoniflorin, oxypaeoniflorin, paeonin, etc.), TAS (consist of astragaloside I-VI, soyasaponin, etc.), TFA, astragalus polysaccharides and so on. The previous results from our laboratory showed that TGP was effective against ALI induced by CCl4, D-galactosamine (D-GalN) and BCG+LPS in mice and chronic liver[1]. In vivo and in vitro, TGP showed obvious anti-inflammatory and antioxidative activities in other diseases besides in liver disease. For example, it was found that treatment of AA rats with TGP (50 mg/kg, ig (14-28 d)) could inhibit the elevated level of MDA and NO, and upregulated the lowered activities of SOD and GSH-px[14,15]. In vitro, TGP could scavenge OH· and O2[16,17]. It was reported[13] that TAS could protect liver from chemical injury induced by CCl4, D-GalN and acetaminophen in mice. TAS could impede the elevation of ALT level, decrease the MDA content and increase the GSH concentration in mice liver homogenate. Obvious improvements of histologic changes were also observed. In vitro, TAS (0.75 μmol/L-0.18 mmol/L) could decrease elevated ALT level in hepatocytes separated from rats. Previous studies of our institute showed that TAS had an antinociceptive effect on formalin test in mice that related to its inhibitory effect on the production of NO. Besides, astragalus polysaccharide was found to have immunoregulatory activity and was used in various kinds of immunologic diseases[17]. In our previous study, the optimum proportion of herbs in FFHQ prescription was obtained by uniform design in ALI mice. On the basis of the optimum proportion, FFHQ extracts were produced with the method stated in the part of “Drugs and materials”. In the present study, the two kinds of ALI models in mice were successfully established, namely immunologic ALI model induced by BCG+LPS and chemical ALI model induced by CCl4 to observe the protective effects and its probable mechanisms of FFHQ.
CCl4 is a well-known hepatotoxic chemical[8,10,18,19]. The main cause of ALI by CCl4 is free radicals of its metabolites. By the activation of liver cytochrome P-450, CCl4 generates methyltrichloride radicals (CCl3), which are highly unstable and immediately react with membrane components. They form covalent bonds with unsaturated fatty acids, or take a hydrogen atom from the unsaturated fatty acids of membrane lipids, resulting in the production of chloroform and lipid radicals. The lipid radicals react with molecular oxygen, which initiates peroxidative decomposition of phospholipids in the endoplasmic reticulum. The peroxidation process results in the release of soluble products that may affect cell membrane. Cell membrane integrity is broken and the enzymes (such as ALT, AST, etc.) in cell plasma leak out. The free radicals and its triggered lipid peroxidation were involved in the main mechanisms by which CCl4 induced ALI[20-25]. MDA was one of the main lipid peroxidation products, its elevated levels could reflect the degrees of lipid peroxidation injury in hepatocytes. However, SOD is a scavenger of peroxide anion radicals[26], which could inhibit the initiation of lipid peroxidation by free radicals; GHS-px could particularly catalyze the reductive action of GSH to H2O2 to protect the integrity of plasma membrane and functions. The present study showed that level of serum ALT, AST and the content of MDA in liver homogenate increased in the model group mice and the activities of SOD and GSH-px decreased correspondingly. FFHQ decreased the elevated level of ALT and AST, markedly inhibited the increase of MDA level and upregulated lower level of the activities of SOD and GSH-px in different extents, in a dose-dependent manner. These results indicated that median and high doses of FFHQ had potential action against lipid peroxidation, and this effect perhaps is the main mechanism of protection on ALI. The results were consistent with that TGP and TAS showed anti-inflammatory and antioxidate activities in our previous and in the studies of others.
Injection of BCG followed by LPS is useful for the creation of experimental models of immunologic ALI[11,27,28]. In the present study, immunologic ALI in mice was successfully induced by BCG+LPS. On this basis, administration of FFHQ in vivo resulted in marked reduction of liver injury, as demonstrated by significant reduction of the serum transaminase concentration and amelioration of the severe hepatic pathologic abnormalities. Meanwhile, FFHQ decreased MDA content and increased GSH-px and total SOD activities in liver homogenate, in a dose-dependent manner. Furthermore, FFHQ significantly reduced TNF-α and IL-1 production in serum, in a dose-dependent manner. In the present study, the effects of FFHQ on two models in mice were investigated first. The results showed that FFHQ deceased MDA content in liver homogenate, meanwhile, SOD and GSH-px activities rose significantly. Those results are in accordance with the findings of FFHQ’s antioxidant properties.
TNF-α is a multifunctional cytokine mostly secreted by inflammatory cells and has been implicated in a number of liver diseases. TNF-α has been proven to be the key mediator and cytokine in the destruction of hepatocyte in human liver diseases[29,30]. Many previous studies have showed that TNF-α could mediate cell injuries in liver caused by alcoholism, endotoxin, reperfusion, primary graft nonfunctional and graft rejection and so on, and the activity of TNF-α was positively related with the extent of liver necrosis[28-31]. At the same time, TNF-α can activate nuclear transcription factor-kappa B of hepatocytes, Kupffer cells and endotheliocyte, which increases expression of intercellular adhesion molecule-1, vascular-cell adhesion molecule-1 and selection, these inflammatory factors further impel the inflammatory injury of hepatocytes[32,33]. The present study is in accordance with the reported results. In the two ALI models in mice, serum TNF-α level in model groups was significantly higher than that in control groups. IL-1 is another critical inflammatory mediator and cytokine in ALI. Although IL-1 itself has no damage on liver, its elevation could stimulate inflammatory cells to excrete many other cytokines including TNF-α, IL-6 and IL-8, which contribute to ALI[34]. Our data provided further evidence for the role of cytokines including TNF-α and IL-1 during ALI. Serum level of TNF-α and IL-1 elevated significantly in model group in immunologic ALI mice model. Median and high doses of FFHQ significantly reduced the elevated level of TNF-α and IL-1 in mice serum. Therefore, inhibition of pro-inflammatory mediator and cytokines is partly the mechanisms of FFHQ protective effect on ALI.
In the two model experiments, histologic changes, such as hemorrhage and necrosis in hepatic lobules, inflammatory infiltration of lymphocytes and Kupffer cells around the central vein, were simultaneously improved in FFHQ treatment groups. All results mentioned above suggested that FFHQ may not only be an anti-inflammatory agent, but also be used as an antioxidative therapy for ALI in mice.
In summary, the present study further demonstrated that inflammatory reaction, free radicals and its triggered lipid peroxidation are main pathologic characteristics of ALI. FFHQ has protective effect either on chemical ALI in mice or on immunologic ALI in mice. The mechanisms of FFHQ on ALI may be related to its immunoregulatory properties and antioxidant, such as free radical scavenging, increased SOD, GSH-px activities and proinflammatory mediators. To conform to the modernization of TCM, the study on active components about the prescription of FFHQ needs to be developed further. Both experiments to extract, isolate and identify the active components about the prescription of FFHQ and studies on the mechanisms involved are now in progress.