Published online May 21, 2005. doi: 10.3748/wjg.v11.i19.2922
Revised: May 26, 2004
Accepted: June 24, 2004
Published online: May 21, 2005
AIM: To investigate the effect of various concentrations of tetrandrine on activation of quiescent rat hepatic stellate cells (HSCs) and transforming growth factor-β (TGF-β) signaling in vitro.
METHODS: HSCs were isolated from rats by in situ perfusion of liver and 18% Nycodenz gradient centrifugation, and primarily cultured on uncoated plastic plates for 24 h with DMEM containing 20% fetal bovine serum (FBS/DMEM) before the culture medium was substituted with 2% FBS/DMEM for another 24 h. Then, the HSCs were cultured in 2% FBS/DMEM with tetrandrine (0.25, 0.5, 1, 2 mg/L, respectively). Cell morphological features were observed under an inverted microscope, smooth muscle-α-actin (α-SMA) was detected by immunocytochemistry and image analysis system, laminin (LN) and type III procollagen (PCIII) in supernatants were determined by radioimmunoassay. TGF-β1 mRNA, Smad 7 mRNA and Smad 7 protein were analyzed with RT-PCR and Western blotting, respectively.
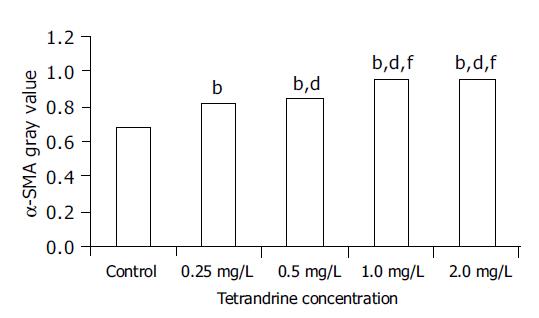
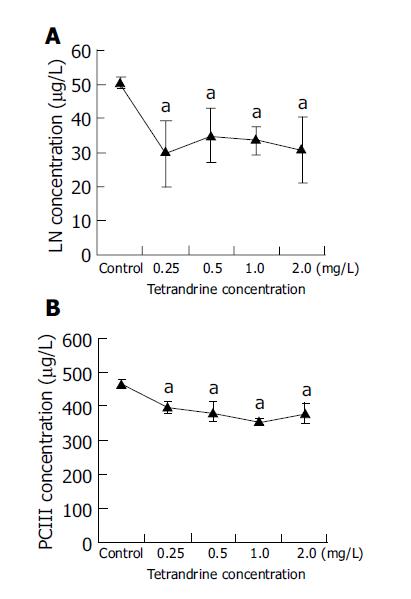
RESULTS: Tetrandrine at the concentrations of 0.25-2 mg/L prevented morphological transformation of HSC from the quiescent state to the activated one, while α-SMA, LN and PCIII expressions were inhibited. As estimated by gray values, the expression of α-SMA in tetrandrine groups (0.25, 0.5, 1, 2 mg/L) was reduced from 21.3% to 42.2% (control: 0.67, tetrandrine groups: 0.82, 0.85, 0.96, or 0.96, respectively, which were statistically different from the control, P<0.01), and the difference was more significant in tetrandrine at 1 and 2 mg/L. The content of LN in supernatants was significantly decreased in tetrandrine groups to 58.5%, 69.1%, 65.8% or 60.0% that of the control respectively, and that of PCIII to 84.6%, 81.5%, 75.7% or 80.7% respectively (P<0.05 vs control), with no significant difference among tetrandrine groups. RT-PCR showed that TGF-β1 mRNA expression was reduced by tetrandrine treatments from 56.56% to 87.90% in comparison with the control, while Smad 7 mRNA was increased 1.4-4.8 times. The TGF-β1 mRNA and Smad 7 mRNA expression was in a significant negative correlation (r = -0.755, P<0.01), and both were significantly correlated with α-SMA protein expression (r = -0.938, P<0.01; r = 0.938, P<0.01, respectively). The up-regulation of Smad 7 protein by tetrandrine (1 mg/L) was confirmed by Western blotting as well.
CONCLUSION: Tetrandrine has a direct inhibiting effect on the activation of rat HSCs in culture. It up-regulates the expression of Smad 7 which in turn blocks TGF-β1 expression and signaling.
- Citation: Chen YW, Wu JX, Chen YW, Li DG, Lu HM. Tetrandrine inhibits activation of rat hepatic stellate cells in vitro via transforming growth factor-β signaling. World J Gastroenterol 2005; 11(19): 2922-2926
- URL: https://www.wjgnet.com/1007-9327/full/v11/i19/2922.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i19.2922
Hepatic stellate cells (HSCs) belonging to the nonparenchymal cell type in Disse space undergo morphological and biochemical transformation, which is quite a complex process of activation and evolution, into myofibroblasts in various liver injuries. The activated HSCs are the main source of extra cellular matrix (ECM). Many profibrogenic cytokines[1,2] can activate HSCs, and among them the transforming growth factor-β1 (TGF-β1) is the master factor for promoting HSC activation, ECM synthesis, and excretion of other profibrogenic factors[3-5]. Abnormally elevated TGF-β1 level and altered intercellular signaling bear a close relation to persistent HSC activation and higher function[4-7].
Both experimental and clinical research have shown that tetrandrine, a bisbenzyl isoquinolone alkaloid derived from Stephania tetrandra S. Moore, a traditional Chinese herb medicine, could exert an anti-inflammation effect on injured liver, attenuate ECM deposition, and inhibit hepatic fibrosis. In order to further investigate the possible mechanism involved, we examined the effects of various dosages of tetrandrine on HSCs and also the expression of laminin (LN), type III procollagen (PCIII), smooth muscle-α-actin (α-SMA), TGF-β1 and its downstream signal transduction components, Smad 7, to elucidate the influence of tetrandrine on activation of HSCs and TGF-β1 signaling.
Male Sprague-Dawley rats weighing 400-500 g were purchased from Shanghai Laboratory Animal Center, Chinese Academy of Sciences. They received normal chow and water ad libitum.
Tetrandrine (molecular formula C38H42N2O6) purchased from Shanxi Huike Botanical Development Co., Ltd, was dissolved in ethanol at 1 mg/mL, diluted and added to the culture medium. The final concentration of ethanol was lower than 0.2%.
DMEM was purchased from Gibco. Nycodenz, pronase E, type IV collagenase and mouse α-SMA monoclonal antibody were purchased from Sigma. Two-step immunocytochemistry detection reagent was purchased from Antibody Diagnostica Inc. LN and PCIII radioimmunoassay kits were purchased from the Institute of Naval Medicine, Shanghai. TRIzol reagent, Moloney murine leukemia virus reverse transcriptase (M-MLV) and rabbit Smad 7 polyclonal antibody were purchased from Invitrogen, Promega, and Santa Cruz, respectively.
HSCs were isolated and cultured as described by Friedman and Roll[8] with some modifications. Briefly, the rats were anesthetized with an intraperitoneal injection of pentobarbital. After cannulation into the portal vein, the liver was perfused with calcium-free balanced salt solution, salt solution containing 0.5 mg/mL collagenase, salt solution containing 0.5 mg/mL collagenase and 1 mg/mL pronase E in turn respectively. Then the liver was taken out, cut into small pieces and incubated in solution containing 0.5 mg/mL collagenase. After being washed by repeated suspension and centrifugation, HSCs were purified by density gradient centrifugation with 18% Nycodenz. HSCs were collected from the top layer, washed and suspended in DMEM supplemented with 20% fetal bovine serum (FBS) at a density of 1×106 cell/mL, and seeded on uncoated 24- and 6-well plastic plates at 1×105/cm2 supplemented with 20% FBS/DMEM for 24 h. Then HSCs were subjected to tetrandrine treatment after cultivation in 2% FBS/DMEM for another 24 h. More than 90% of isolated HSCs were viable as assessed by tryphan blue exclusion and consisted of more than 90% of HSCs as determined by direct cell counting under a phase-contrast microscope and intrinsic vitamin A autofluorescence[9].
HSCs were cultured on 24-well plates without or with tetrandrine (0.25, 0.5, 1, 2 mg/L). After being incubated for 3 d, the supernatant was collected and preserved at -20 °C for further assay. Cells were fixed with ethanol/acetic acid, and α-SMA antibody, horse radish peroxidase-conjugated secondary antibody and diaminobenzidine were added sequentially according to the standard protocol. Semi-quantitative assessment of protein expression was performed using a pathological image analysis system. The expression of α-SMA was estimated by gray value.
HSCs were seeded on 6-well plates and treated as above. Total RNA was extracted with TRIzol reagent. For RT-PCR, 1 μg total RNA was reverse transcribed with M-MLV according to manufacturer’s instructions. cDNAs were amplified using specific sets of primers for TGF-β1 (ggactctccacctgcaagac, ccccagaaatcatcgagac) and Smad 7 (ctgtgttgctgtgaatcttac, gctgtaggccttttcatagt). The PCR procedure for TGF-β1 consisted of 30 cycles of denaturation at 94 °C for 30 s, annealing at 53 °C for 30 s, extension at 72 °C for 30 s, with initial denaturation of sample cDNAs at 94 °C for 5 min before PCR and additional extension period of 10 min after the last cycle. A touch down PCR procedure was performed for Smad 7 cDNA amplification. After denaturation at 94 °C for 5 min, 10 cycles were run with an initial annealing temperature at 58 °C, which was 0.5 °C lower after each cycle before 25 cycles of denaturation at 94 °C for 40 s, annealing at 53 °C for 40 s, extension at 72 °C for 40 s, and additional extension period of 10 min after the last cycle. In parallel, PCR reactions were performed with primers coding for the housekeeping gene, GAPDH, to control for equal amounts of template cDNAs. Five microliters of 20 μL total PCR reaction was analyzed in a 20 mg/g agarose gel with a 100-bp DNA marker. Densitometric analysis of PCR products was performed by the computer software, SmartViewer (Shanghai Furi Science and Technology), and standardized by the GAPDH.
HSCs, which were cultured on 6-well plates with or without tetrandrine (1 mg/L) for 3 d, were washed twice with Hanks’ balanced salt solution and lysed directly in SDS loading buffer. Cell lysates (5 μg of protein) were subjected to SDS-polyacrylamide gel electrophoresis on a 0.1 g/g gel and then transferred to a nitrocellulose membrane. The membrane was incubated with antibody to Smad 7 (diluted 1:500) at 4 °C for 12 h. After being vigorously washed, the membrane was incubated with horse radish peroxidase-conjugated secondary antibody (diluted 1:2000). The membrane blot was developed with diaminobenzidine.
LN and PCIII in supernatant were measured with radioimmunoassay kits according to manufacturer’s instructions.
All data were expressed as mean±SD. Statistical significance for the difference between the groups was assessed using one-way ANOVA test. P<0.05 was considered statistically significant.
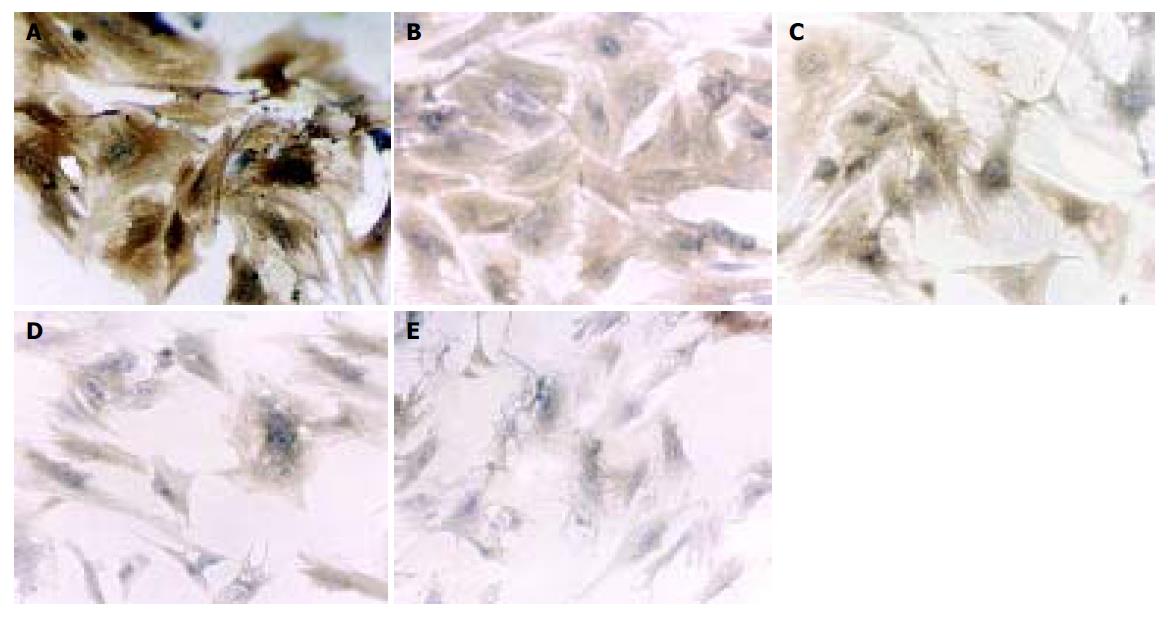
HSCs cultured for 5 d exhibited flattened and membranous processes, representing a myofibroblastic morphology (Figure 1A), while those treated with tetrandrine (0.25, 0.5, 1, 2 mg/L) showed a more slender, spindle stellate cell shape (Figures 1B-1E), which was similar to the appearance of quiescent HSCs.
In the control group, HSCs cultured for 5 d were strongly positive for α-SMA (Figure 1A), which was weaker in tetrandrine-treated groups in varying degrees according to the doses used (Figures 1B-1E). Image analysis showed a statistical difference between the control and tetrandrine groups (P<0.01), and the difference was more significant in tetrandrine at 1 and 2 mg/L (Figure 2).
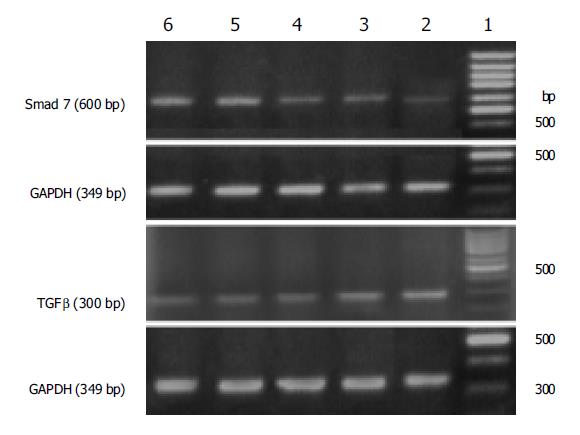
TGF-β1 mRNA expression of HSCs was suppressed from 56.56% to 87.90% in tetrandrine groups (0.25, 0.5, 1, 2 mg/L) when compared with that of the control (P<0.01), while the up-regulated Smad 7 mRNA expression in tetrandrine groups reached 2.4-5.8 times that of the control. A dosage-dependent effect was observed at 0.25-1 mg/L (P<0.01), but no difference at 1 and 2 mg/L was demonstrated (F = 0.394, P = 0.564) (Table 1 and Figure 3). TGF-β1 and Smad 7 mRNA expressions showed a clear negative correlation (r = -0.755, P<0.01), and both were significantly correlated with α-SMA protein expression (r = -0.938, P<0.01; r = 0.938, P<0.01, respectively).
Compared with the control group, the content of LN in HSC culture medium was reduced in tetrandrine groups (0.25, 0.5, 1, 2 mg/L) to 58.5%, 69.1%, 65.8% or 60.0%, respectively (P<0.05), and that of PCIII to 84.6%, 81.5%, 75.7% or 80.7%, respectively (P<0.05), whereas there was no difference among the groups with various concentrations of tetrandrine (Figure 4).
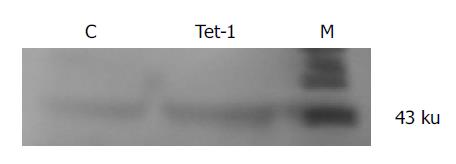
Immunoblot analysis indicated that HSCs after 5 d of culture expressed Smad 7, which was markedly increased by tetrandrine (1 mg/L) treatment. This was consistent with RT-PCR results (Figure 5).
Tetrandrine is a bisbenzyl isoquinolone alkaloid extracted from Stephania tetrandra S. Moore, a traditional Chinese herb medicine. With the progression of basic researches and clinical experiments in recent years, tetrandrine has been demonstrated to be effective in anti-inflammation, immuno-modulation, reversion of cardiac and vascular remodeling, inhibition of pulmonary vessels and airway smooth muscle contraction, suppression of tumor proliferation and multi-drug resistance, and so on[10-12]. Anti-fibrogenesis have long been an important field. Over the years, more experimental animal and clinical studies have shown that tetrandrine has anti-fibrogenic actions, such as inhibition of inflammatory reaction of injured liver, attenuation of ECM deposition, decrease in serum PCIII and LN levels, and inhibiting proliferation and collagen synthesis of fibroblasts or HSCs induced by platelet-derived growth factor. Some earlier studies showed a direct inhibiting effect of tetrandrine on the proliferation of vascular smooth muscle cells and pulmonary fibroblasts[13,14]. More recently, Lee et al[15], showed that tetrandrine inhibited tissue inhibitor of metalloproteinase expression and thus attenuate liver fibrosis, while the study of Park et al[16], revealed that α-SMA, the marker of activated HSCs, was significantly reduced when primary cultured HSCs were treated with tetrandrine (10 mg/L), and much higher concentrations of tetrandrine induced apoptosis in HSCs[17]. However, little is known how tetrandrine affects HSC activation, the key process in the progression of liver fibrosis, and the possible mechanism involved. It is true when it comes to a lower concentration of tetrandrine of less than 10 mg/L. This seems to be more important when realizing its cytotoxicity. In this study, using the most popular cell model of HSC activation[2], we disclosed that tetrandrine could suppress the morphological transformation of HSCs from the quiescent type to activated one, and decrease the expression of α-SMA. Likewise, the production of LN and PCIII and expression of TGF-β1 mRNA were diminished. Our results strongly demonstrated the direct inhibiting effect of tetrandrine on HSC activation and transformation to myofibroblast. Additionally, our data revealed the influence of tetrandrine on inhibitory signaling molecule (Smad 7) for TGF-β. All the findings enriched our knowledge of the anti-fibrogenic mechanism of tetrandrine.
In the course of liver fibrosis, activated HSCs are the most important source of TGF-β1, which in turn induces and accelerates the activation of quiescent HSCs in an autocrine and paracrine manner, promotes ECM production, and results in progression of liver fibrosis. It has been widely accepted that TGF-β1 is the most potent profibrogenic mediator in liver fibrosis[2-4], and thus to inhibit TGF-β1 function is of great importance and effective in anti-fibrogenic strategy[4,18-20]. The signals of TGF-β mainly go through its receptors and downstream Smad proteins to HSCs[21]. Smads, including Smad 1-Smad 8, are the intercellular components of the signal pathway of TGF-β superfamily. Smads interact with type I and type II TGF-β receptors and directly or indirectly transduce or modulate TGF-β signals into nuclei[5,6]. It has been reported that the balance between Smads may be the key issue in maintaining normal TGF-β functions[5,21], and Smad 7 is the main negative feedback regulator of TGF-β signaling in HSCs[6]. However, in activated HSCs, TGF-β-induced Smad 7 expression was diminished in an unknown way and this was thought to be strongly associated with the overwhelming profibrogenic action of TGF-β following liver injury. Therefore, an attempt to up-regulate Smad 7 expression might be a promising way to reduce TGF-β excretion, restore normal signal regulation, and inhibit HSC activation with resulting abnormal functions.
Our study indicated that the activation of HSCs was significantly inhibited by tetrandrine, as shown by the delayed morphologic transformation, diminution of α-SMA and TGF-β1 mRNA expression, decrease in LN and PCIII production, increase in Smad 7 expression. Statistical analysis showed a close correlation among the changes of α-SMA, TGF-β1 mRNA and Smad 7. These data suggested that tetrandrine inhibited activation of HSCs via down-regulation of TGF-β1 expression. However, this may not be the only mechanism, for it might be the result of inhibition of HSC activation via some other pathways instead of TGF-β1 signaling[22]. Smad 7, both at mRNA and protein levels, was remarkably increased in comparison with lowered TGF-β1, suggesting that Smad 7 plays an important role in HSC inactivation, in addition to the result of diminution of TGF-β1. Tetrandrine may inhibit activation of HSCs by up-regulation of Smad 7 with resulting blockage of TGF-β gene transcription. This view is supported by recently reported findings that up-regulation of Smad 7 or blockage of TGF-β1 signaling resulted in inhibition of HSC activation[23,24].
HSC activation is a complicated process, which is even true when it comes to in vivo studies. The reasons why the feedback regulation of TGF-β signaling in normal liver cell is maintained and why such a regulation is lost in the pathological process of liver fibrosis are still not fully understood at present. Although in this study we revealed that tetrandrine could influence TGF-β1 and its signaling through Smads, more efforts should be made to further investigate the mechanisms involved and the interaction with other factors in their signal transduction pathways.
| 1. | Reeves HL, Friedman SL. Activation of hepatic stellate cells--a key issue in liver fibrosis. Front Biosci. 2002;7:d808-d826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 338] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 2. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1597] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 3. | Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793-d807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 326] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 4. | Shek FW, Benyon RC. How can transforming growth factor beta be targeted usefully to combat liver fibrosis? Eur J Gastroenterol Hepatol. 2004;16:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Bauer M, Schuppan D. TGFbeta1 in liver fibrosis: time to change paradigms? FEBS Lett. 2001;502:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Nakao A, Afrakhte M, Morén A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1433] [Cited by in RCA: 1474] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 7. | Dooley S, Streckert M, Delvoux B, Gressner AM. Expression of Smads during in vitro transdifferentiation of hepatic stellate cells to myofibroblasts. Biochem Biophys Res Commun. 2001;283:554-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Friedman SL, Roll FJ. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal Biochem. 1987;161:207-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 292] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Uyama N, Shimahara Y, Okuyama H, Kawada N, Kamo S, Ikeda K, Yamaoka Y. Carbenoxolone inhibits DNA synthesis and collagen gene expression in rat hepatic stellate cells in culture. J Hepatol. 2003;39:749-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Lai JH. Immunomodulatory effects and mechanisms of plant alkaloid tetrandrine in autoimmune diseases. Acta Pharmacol Sin. 2002;23:1093-1101. [PubMed] |
| 11. | Xie QM, Tang HF, Chen JQ, Bian RL. Pharmacological actions of tetrandrine in inflammatory pulmonary diseases. Acta Pharmacol Sin. 2002;23:1107-1113. [PubMed] |
| 12. | Wang G, Lemos JR, Iadecola C. Herbal alkaloid tetrandrine: fron an ion channel blocker to inhibitor of tumor proliferation. Trends Pharmacol Sci. 2004;25:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Zhang JX, Pegoli W, Clemens MG. Endothelin-1 induces direct constriction of hepatic sinusoids. Am J Physiol. 1994;266:G624-G632. [PubMed] |
| 14. | Reist RH, Dey RD, Durham JP, Rojanasakul Y, Castranova V. Inhibition of proliferative activity of pulmonary fibroblasts by tetrandrine. Toxicol Appl Pharmacol. 1993;122:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Lee SH, Nan JX, Sohn DH. Tetrandrine prevents tissue inhibitor of metalloproteinase-1 messenger RNA expression in rat liver fibrosis. Pharmacol Toxicol. 2001;89:214-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Park PH, Nan JX, Park EJ, Kang HC, Kim JY, Ko G, Sohn DH. Effect of tetrandrine on experimental hepatic fibrosis induced by bile duct ligation and scission in rats. Pharmacol Toxicol. 2000;87:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Zhao YZ, Kim JY, Park EJ, Lee SH, Woo SW, Ko G, Sohn DH. Tetrandrine induces apoptosis in hepatic stellate cells. Phytother Res. 2004;18:306-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Ueno H, Sakamoto T, Nakamura T, Qi Z, Astuchi N, Takeshita A, Shimizu K, Ohashi H. A soluble transforming growth factor beta receptor expressed in muscle prevents liver fibrogenesis and dysfunction in rats. Hum Gene Ther. 2000;11:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Isono M, Soda M, Inoue A, Akiyoshi H, Sato K. Reverse transformation of hepatic myofibroblast-like cells by TGFbeta1/LAP. Biochem Biophys Res Commun. 2003;311:959-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Xu XB, He ZP, Liang ZQ, Leng XS. Obstruction of TGF-beta1 signal transduction by anti-Smad4 gene can therapy experimental liver fibrosis in the rat. Zhonghua GanZangBing ZaZhi. 2004;12:263-266. [PubMed] |
| 21. | Bissell DM, Roulot D, George J. Transforming growth factor beta and the liver. Hepatology. 2001;34:859-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 284] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 22. | Fang LH, Zhang YH, Ma JJ, Du GH, Ku BS, Yao HY, Yun YP, Kim TJ. Inhibitory effects of tetrandrine on the serum- and platelet-derived growth factor-BB-induced proliferation of rat aortic smooth muscle cells through inhibition of cell cycle progression, DNA synthesis, ERK1/2 activation and c-fos expression. Atherosclerosis. 2004;174:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Dooley S, Hamzavi J, Breitkopf K, Wiercinska E, Said HM, Lorenzen J, Ten Dijke P, Gressner AM. Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology. 2003;125:178-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 306] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 24. | Zhao JF, Liu CH, Hu YY, Xu LM, Liu P, Liu C. Effect of salvianolic acid B on Smad3 expression in hepatic stellate cells. Hepatobiliary Pancreat Dis Int. 2004;3:102-105. [PubMed] |