Published online May 21, 2005. doi: 10.3748/wjg.v11.i19.2885
Revised: February 3, 2004
Accepted: April 5, 2004
Published online: May 21, 2005
AIM: To translate Tet-on system into a conditional mouse model, in which hepatitis B or C virus (HBV or HCV) gene could be spatiotemporally expressed to overcome “immune tolerance” formed during the embryonic development and “immune escape” against hepatitis virus antigen(s), an effector mouse, carrying the reverse tetracycline-responsive transcriptional activator (rtTA) gene under the tight control of liver-specific human apoE promoter, is required to be generated.
METHODS: To address this end, rtTA fragment amplified by PCR was effectively inserted into the vector of pLiv.7 containing apoE promoter to create the rtTA expressing vector, i.e., pApoE-rtTA. ApoE-rtTA transgenic fragment (-6.9 kb) released from pApoE-rtTA was transferred into mice by pronucleus injection, followed by obtaining one transgene (+) founder animal from microinjection through PCR and Southern blot analysis.
RESULTS: rtTA transgene which could be transmitted to subsequent generation (F1) derived from founder was expressed in a liver-specific fashion.
CONCLUSION: Taken together, these findings demonstrate that rtTA transgenic mice, in which rtTA expression is appropriately targeted to the murine liver, are successfully produced, which lays a solid foundation to ‘off-on-off’ regulate expression of target gene (s) (e.g., HBV and/or HCV) in transgenic mice mediated by Tet-on system.
- Citation: Xu K, Deng XY, Yue Y, Guo ZM, Huang B, Hong X, Xiao D, Chen XG. Generation of the regulatory protein rtTA transgenic mice. World J Gastroenterol 2005; 11(19): 2885-2891
- URL: https://www.wjgnet.com/1007-9327/full/v11/i19/2885.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i19.2885
Hepatitis C virus (HCV) infection is a global public health problem, with approximately 3% of the world population now infected; HCV infection is the major cause of post-transfusion non-A non-B hepatitis; persistent HCV infection often progresses to chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (HCC), usually more than a decade after initial infection[1,2]. Thus, the development of adequate treatment and prophylactics for HCV infection has been important. Nonetheless, HCV is not infectious in vivo except in primates, a phenomenon that has resulted in the lack of a proper HCV culture system and inbred animal model, which has in turn hampered detailed analysis of viral life cycle and pathogenesis of HCV infection[1,2]. Therefore, establishing in vitro and in vivo valuable models for human HCV infection is of great importance.
Since the first report of transgenic mice generated by injecting DNA into the pronucleus of one-cell mouse embryos, this technique has been immensely useful in creating model organisms for research purposes[3]. A great number of transgenic mouse models created by conventional transgene technology for human viral hepatitis [e.g., hepatitis B virus (HBV) and HCV] have already been established and provided new insights into the pathogenesis of hepatitis and HCC[4-7]. However, one biggest shortcoming of the consistent gene expression system is that the conventional transgene systems provide only “immune tolerant” mice for transgene products, e.g., viral antigene (s), that is to say, the transgenic animals for HBV or HCV are not immunocompetent for the transgene product (s). In the consistent gene expression system, once transferred into embryos, the target gene immediately begins to express viral protein (s) at the early stage of embryo development under the control of the promoter before the formation of immune system. During embryo development, immune cells are stimulated by viral antigen (s) to progressively develop maturation with concurrent “immune tolerance” to virus antigen (s), which makes hepatocyte injury uncertain. Thus, after birth the immune system of organisms cannot recognize the exotic identity of viral antigen (s) and not only in theory but also in fact the liver damage in transgenic mice is not ascertained. In human chronic hepatitis C, hepatocyte injury is not directly caused by HCV infection, but is a consequence of the destruction of infected hepatocytes by cytotoxic lymphocytes[8]. In fact, the immune system plays pivotal roles in pathogenesis of HCV infection[9-15]. The traditional HBV and HCV transgenic mice were assayed to find that antigen gene (s) could express normally, but obviously pathologic changes are not observed in the liver and the serum alanine aminotransferase levels were basically normal, indirectly suggesting that the immune system plays a rather important part in hepatitis pathogenesis[4-7]. So this kind of HCV or HBV transgenic mice is not extremely ideal models suitable for investigating host immune response against HBV or HCV infection and pathogenesis of HBV or HCV infection.
One expected goal of transgene technology is conditional control of target gene, e.g., viral gene (s), expression in a specific tissue/organ during a particular stage of development to mimic viral infections in humans. Therefore, by integrating with the conventional transgene technology, the inducible expression systems for temporal, spatial, and cell-specific control of gene expression in mice provide an approach to tide over the limitation of the stable expression system described above and may be employed to generate immunocompetent transgenic mice with hepatitis B or C.
Heretofore, among the inducible overexpression transgenic systems, the tetracycline-inducible systems, as a reliable excellent tool for stringently reversible (on ⇔ off; ‘off-on-off’ or ‘on-off-on’ regulation is more attractive when verifying the function of a given gene, and would be valuable for stage-specific serial gene regulation in developmental studies), temporal and spatial control of transgene expression, have been successfully and most frequently used in transgenic mouse modeling[16-18]. There are two basic variants: one is the tTA (tetracycline-controlled transactivator) system (“Tet-Off”system)[19] and the other is rtTA (reverse tTA) system (“Tet-On” system)[20]. Based on the characteristics of two systems that work through the opposite mechanism, if a gene is to be kept inactive most of the time and turned on only occasionally, Tet-on system appears to be more appropriate.
Therefore, to well elucidate host immune response against HBV or HCV infection and pathogenesis of HBV or HCV infection, we plan to employ Tet-on system to establish a binary transgenic mouse model in which the conditional expression of HBV or HCV transgene can be tightly regulated in the liver by administration of doxycycline (Dox). To use this system in vivo, it is necessary to generate two sets of transgenic animals. One mouse line expresses the activator rtTA under the control of a liver-specific promoter that targets rtTA expression at the liver. Another set of transgenic animals, in which HBV or HCV transgene expression is under the control of the target sequence for rtTA, harbors the “acceptor” transgenic construct, i.e., TRE-PminCMV-HBV or TRE-PminCMV-HCV. Mating two strains of mice will and should result in the birth of bi-transgenic offspring, allowing in vivo reversible and spatiotemporal control of HBV or HCV transgene expression through addition or without addition of Dox to the food or drinking water of the double-transgenic mice.
The rtTA system has been used successfully in numerous transgenic animal models with a variety of transgenes targeted at various tissues and organs; in Tet-regulated transgenic mice, tissue specificity of target gene expression is conferred by the promoter driving rtTA expression, in other words, defining the site of expression of rtTA determines the site of transgene expression, because the minimal promoter (e.g., tetO-PminCMV in responsive element) itself confers no tissue specificity[16-18,21]. Unfortunately, there is lack of one transgenic mouse line expressing rtTA in a liver-specific manner (http://www.zmg.unimainz.de/tetmouse/)[16-18,21]. Thus, this study was undertaken to generate the transgenic mice expressing regulatory protein rtTA under the control of a liver-specific apoE promoter to lay a solid base for spatiotemporal expression of HBV and/or HCV in transgenic mouse modeling.
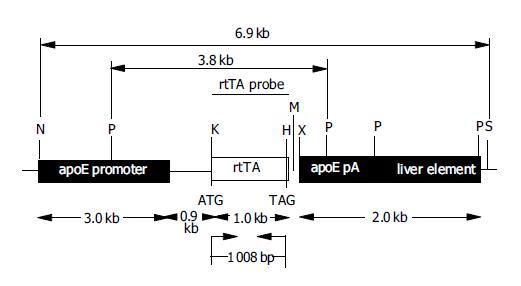
For liver-specific expression of rtTA in vivo, the transgenic construct ApoE-rtTA, containing rtTA under the control of the liver-specific apoE promoter, was constructed (Figure 1). rtTA fragment (774-1781) was amplified by PCR using pTet-on DNA (Clontech), which encodes the regulatory protein rtTA, as template and the rtTA specific primers corresponded to the plasmid pTet-on with the suitable restriction sites KpnI and HpaI incorporated into the forward and reverse primers, respectively. The primers specific for rtTA were rtTA forward primer (rtTA-FP): 5’-CCGGGGTACC ATG TCT AGA TTA GAT AAA AGT-3’ and rtTA reverse primer (rtTA-RP): 5’-TATAGTTAAC CTA CCC ACC GTA CTC GTC-3’ (added restriction sites of KpnI and HpaI were underlined sequentially). PCR reaction conditions were: 30 cycles of 94 °C for 50 s, 58 °C for 50 s, and 72 °C for 1 min 30 s. PCR product (1008 bp) of amplified rtTA was first cloned into pMD18-T (T-vector; Takara) by T/A cloning to give pMD18-T-rtTA, and thereafter sequenced with general sequencing primers M13-47/RV-M (Takara). After confirmed to be identical to the published rtTA sequences (Genbank accession no. U89930), rtTA fragment was released from pMD18-T-rtTA using KpnI and HpaI, and then directionally subcloned into the KpnI and HpaI sites in the polylinker of the expression vector pLiv.7 (9.3 kb) containing a liver-specific human apolipoprotein E promoter (apoE promoter)[22], designated as pApoE-rtTA, followed by identification of PCR and enzyme digestion analysis.
Transgenic mice were generated in F1 zygotes using standard pronuclear injection as previously described[23]. The Kunming mouse line, supplied by Center of Experimental Animals, Zhongshan University, was used as the source of embryos for the micromanipulation and subsequent breeding trials. All transgenic lines were created on the Kunming mouse background. For microinjection, the -6.9-kb fragment of transgene ApoE-rtTA (Figure 1) was separated free from the vector backbone of pApoE-rtTA by NotI and SpeI double digestion. The injected fragments of ApoE-rtTA were isolated and purified using the QIA quick gel extraction kit (Qiagen), diluted to a final concentration of 2 μg/mL DNA injection buffer (10 mmol/L Tris/0.1 mmol/L EDTA, pH 7.4), and microinjected into the pronuclei of one cell-stage fertilized embryos [Kunming mouse (♀) ×Kunming (♂)]. Then 20-25 injected DNA fertilized eggs that survived microinjection were implanted into the oviducts of one pseudopregnant recipient Kunming mouse as described[23] 2-3 h after injection or on the next day. Potential transgenic founder animals were weaned at 3 wk of age, and identified by screening mouse tail genomic DNA prepared with standard protocols[24] for the presence of ApoE-rtTA transgene using PCR, and confirmed by standard Southern blotting analysis with horseradish peroxidase (HRP)-labeled rtTA DNA as a probe.
PCR was performed on tail genomic DNA preparations to screen which mice had ApoE-rtTA integrated into their genome. Amplification reactions for genotype animals used the oligonucleotide pairs rtTA-FP/rtTA-RP (see above) specific for rtTA coding region (see Figure 1 for their positions) to amplify a -1-kb fragment. Reaction conditions were: 30 cycles of 94 °C for 50 s, 62 °C for 50 s, and 72 °C for 1 min 30 s. The positive control for each PCR reaction used 100 ng of ApoE-rtTA construct DNA. Genomic DNA from wild-type mice was amplified as a reaction control (e.g., negative control). DNA samples were considered positive for a particular transgene, if a band of the predicted size in the test sample was present with no amplification occurring in the control sample.
The north2south® direct HRP labeling and detection kit (Pierce) is a complete system for labeling and chemiluminescent detection of nucleic acids in Northern and Southern blot applications. This one-step labeling and hybridization system combined with a novel enhanced luminol substrate for HRP ensures rapid and consistent results with sensitivity equal to or exceeding 32P.
To further confirm presence of ApoE-rtTA in the transgenic mouse genome, Southern blots were performed by standard techniques[24] and following the manufacturer’s instructions of north2south® direct HRP labeling and detection kit. Briefly, 10 μg of tail genomic DNA from PCR-positive pups was digested overnight with PstI, fractionated by electrophoresis through 0.8% agarose gels in Tris-borate-EDTA (TBE) buffer (90 mmol/L Tris-borate, 2 mmol/L EDTA, pH 8.0), transferred onto a positively charged nylon membrane (Schleicher & Schuell, Keene, NH), which was not fixed with UV crosslinking, by alkaline transfer, and subjected to prehybridization and hybridization with probe (see Figure 1 for its position) of -1-kb HRP-labeled KpnI–HpaI fragment from pApoE-rtTA synthesized according to the protocol of probe labeling in kit. After stringent washes, the membranes were then subjected to chemiluminescence analysis with a commercial north2south® direct HRP labeling and detection kit. The chemiluminescence-treated membranes were then exposed to X-ray film (X-Omat AR-5, Eastman Kodak Company, Rochester, NY), usually for 1-10 min at room temperature. Genomic DNA from normal Kunming mice was used as a negative control, while a 3.9-kb fragment excised from the 6.9-kb transgenic ApoE-rtTA by PstI digestion was employed as the positive control for Southern blots.
At 6-8 wk of age, founder mice were backcrossed with normal Kunming mice to generate F1. The genotypes of the founder progeny were analyzed for inheritance of the transgene by PCR performed using the rtTA-FP/RP primers (see above for details) and genomic DNA isolated from tail biopsy samples of 4-wk founder progeny. The PCR protocols for rtTA were noted above.
RNA extraction The isolation of total RNA from the different tissues of 5-6-mo old F1 PCR-positive offspring of founder (s), and non-transgenic littermates of F1 PCR-positive transgenic pups and normal mice as negative controls was performed using the RNeasy Mini Kit (Qiagen) following the manufacturer’s recommendations. Purified RNA was eluted in a final volume of 50 μL DNA-free water and aliquots were stored at -80 °C with 2 μL of RNasin.
RT-PCR is thought to be the most sensitive method for the detection of RNA, but contamination of DNA originated from animal genome results in false positivities. In this study, we used one-step mRNA selective PCR kit (Version 1.1) (TaKaRa) that could only detect target mRNA distinguished from genomic DNA of host cells, by using dNTP analogs[25,26]. The dNTP/analogs were incorporated into cDNA formed with mRNA as a template at the reverse transcription (RT) step. The cDNA/mRNA hybrid was denatured at about 85 °C, but genomic DNA was not. The dNTP/analog incorporated into cDNA was selectively amplified at the next PCR step. Using this system, there is the possibility that only target mRNA is detected, even if there is contamination by genomic DNA.
A specific system for the amplification of mRNA used was one-step mRNA selective PCR kit (version 1.1) (TaKaRa). RT-PCR was carried out as recommended by the manufacturer (Takara) with minor modifications. Briefly, it was carried out in a volume of 50 μL including 25 μL 2×mRNA selective PCR buffer I, 10 μL 25 mmol/L MgCl2, 5 μL 1 mmol/L dNTP/analog mixture each, 1 μL RNase inhibitor (40 U/mL), 1 μL AMV reverse transcriptase XL (5 U/mL), 1 μL AMV-optimized Taq (5 U/mL). In the reaction volume of 50 μL, -1 μg total RNA was used to synthesize the single-stranded cDNA with AMV reverse transcriptase XL (Takara) in one-step RT-PCR. The oligonucleotide primers used for RT-PCR were rtTA-FP/RP primers (see above for details) (PCR product size: -1 kb). RT-PCR amplification was carried out as follows: 30 min at 50 °C for RT, denaturation for 5 min at 85 °C and then a succession of 35 cycles as follows: 1 min at 85 °C, 1 min at 58 °C, 90 s at 72 °C, and a final extension at 72 °C for 10 min.
The integrity of each tissue RNA sample was checked by RT-PCR with primers for the human β-actin gene, used as an internal standard (sense: 5’- GAT ATC GCT GCG CTG GTC GT -3’ and antisense: 5’- CGG AAC CGC TCG TTG CCA AT -3’), which produced a 758-bp fragment. For the detection of β-actin mRNA, 30 cycles of one-step RT-PCR were carried out (30 min at 50 °C for RT, and then a succession of 30 cycles as follows: 85 °C for 1 min, 62 °C for 1 min, and 72 °C for 1 min).
Equal quantities (-1 μg) of total RNA were tested in each reaction of RT-PCR. The negative control reactions including reagent control without reverse transcriptase to ensure that RT-PCR was RNA-dependent, negative control I (total RNA from the normal Kunming mouse), and negative control II (total RNA from the non-transgenic littermates of F1 PCR-positive offspring derived from founder (s)) were performed simultaneously under identical conditions. All experiments were performed in triplicate.
To express rtTA in a liver-specific fashion in vivo, we constructed a fusion gene of the apoE promoter (3.0 kb), which targeted expression of rtTA transgene at the murine liver, and rtTA gene (1008 bp) (Figure 1) to prepare the rtTA expression vector, i.e., pApoE-rtTA.
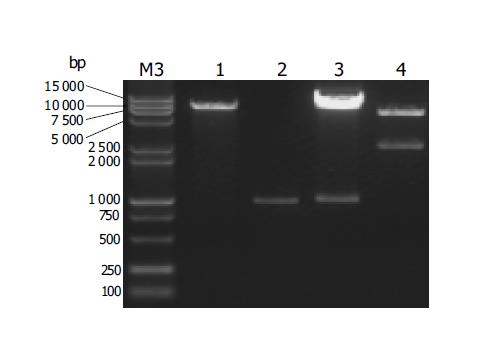
rtTA fragment (-1 kb) was amplified by PCR (data not shown), subsequently inserted into pMD18-T (T-vector) to prepare pMD18-T-rtTA screened from many clones by PCR (data not shown), and thereafter sequenced (data not shown). After confirming its sequence, rtTA fragment was removed from pMD18-T-rtTA using KpnI and HpaI, isolated and purified (Figure 2), and then directionally subcloned into KpnI and HpaI sites of the expression vector pLiv.7 (8.3 kb) linearized with KpnI and HpaI (Figure 2) to generate pApoE-rtTA. The desired recombinant pApoE-rtTA (9.3 kb) would also be confirmed by electrophoresis map of enzyme digestion (Figure 2) and by sequencing of the sequence in the frame of ApoE-rtTA transgene (data not shown). The expected pApoE-rtTA would release two fragments of -1 and 8.3 kb after digested by KpnI and HpaI (Figure 2). In addition, two predicted fragments, -6.9 kb ApoE-rtTA transgene and 2.4 kb vector backbone, were excised from pApoE-rtTA with NotI and SpeI (Figure 2).
For microinjection, a -6.9-kb fragment of ApoE-rtTA transgene was excised from pApoE-rtTA with NotI and SpeI, isolated and purified (data not shown). The structure and components of ApoE-rtTA transgene construct are fully demonstrated in Figure 1. For the liver-specific rtTA expression, the construct ApoE-rtTA was generated to express target gene under the control of the liver-specific apoE promoter. A 6.9-kb transgenic construct used for microinjection was released from pApoE-rtTA via digestion with NotI and SpeI. The transgenic construct contains the human apoE regulatory region (3.0 kb), i.e., an apoE promoter for liver-specific expression of gene, followed by an apoE intron (0.9 kb), and human apoE gene poly(A) signal (apoE pA, 254 bp) and a liver element (1.7 kb) ensuring efficient transgene transcription in the liver. The 1008-bp rtTA fragment [encoding the regulatory protein rtTA with the indicated translation initiation (ATG) and termination (TAG) sites] was inserted just after the intron, followed by the downstream regulatory sequence of human apoE pA and a liver element. The restriction sites are: H, HpaI; K, KpnI; M, MluI; P, PstI; N, NotI; S, SpeI; X, XhoI. The restriction enzyme(s) used in Southern blot hybridization are shown in boldface. The positions of the hybridization rtTA probe (black bar) and predicted size of fragment detected by the probe of HRP-labeled rtTA fragment (-1 kb), the primers specific for the rtTA used in PCR amplification (small arrows) and expected size of the PCR products are indicated. For Southern blot analysis, the genomic DNA samples were digested with PstI; as the probe, -1 kb HpaI- KpnI fragment of ApoE-rtTA construct was used. At the bottom of diagram, the fragment size of the individual sequence in the transgenic construct is shown. The construct map is not drawn to the scale.
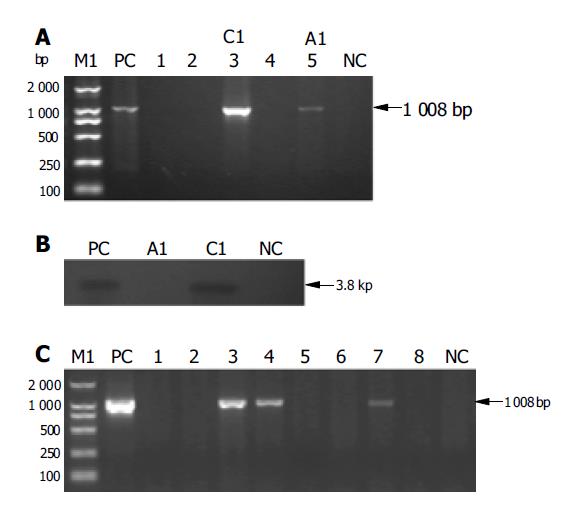
The -6.9 kb ApoE-rtTA was transferred into mice by pronucleus injection. Of 357 embryos transferred to recipient females, 55 embryos developed to term. Among 55 potential founders PCR analysis revealed two positivities (Figure 3A), i.e., C1 and A1, but Southern blot analysis showed one positivity (e.g., C1) (Figure 3B), carrying ApoE-rtTA transgene. Therefore, one founder animal was attained. Fifty-five offspring were individually analyzed by PCR for the genomic integration of transgene with tail biopsy-derived DNA of the potential transgenic founder mice and rtTA primers shown in Figure 1. The results were compared with those obtained with DNA from a negative control (NC) wild-type mouse (lane NC) and positive control (PC) ApoE-rtTA DNA (lane PC). Lanes 1-5, the genomic DNA of the representative five animals were analyzed out of 55 F0 pups born in PCR reaction, the data on the rest of non-transgenic littermates were not shown. The molecular weight of amplified rtTA fragment band was ascertained as -1 kb calculated by the amplified band in lane PC and by the migration of standard DNA molecular weight markers [DL2000 DNA marker (2000, 1000, 750, 500, 250, 100 bp) (TaKaRa)] (lane M1), size markers are shown to the left. The arrow indicates the positions of PCR products amplified by the primers shown in Figure 1. Lanes 3 (C1) and 5 (A1) show the amplified 1008-bp band. Genomic DNA (10 μg) from PCR positive founder mice (e.g., C1 and A1) and a negative control (NC) normal mouse (lane NC) was digested with Pst I and used for Southern analysis with an rtTA probe shown in Figure 1. A 3.8-kb fragment (Figure 1) isolated from transgenic ApoE-rtTA construct by Pst I digestion was used as a positive control (lane PC). Desired fragment size detected by rtTA probe, as calculated by the hybridized band in lane PC and the relative positions of fragments of known size in bp [M4: λ - EcoT14 I digest Marker (TaKaRa)] (data not shown), was indicated at the right of the blot. This is a representative Southern blot from three separate experiments that yielded similar results.
In addition, to determine whether the transgene ApoE-rtTA was passaged to the next generation, founder (C1) was back-crossed to the parental mouse strain to give F1 generation. PCR analysis of F1 offspring (8), derived from founder C1, showed that the percentage of transgenic animals in the progeny was 37.5% (3/8) (Figure 3C). PCR analysis was performed to examine the possibility that the foreign transgene ApoE-rtTA was transmitted from founder C1 to subsequent generation (F1, eight littermates) from the mating of founder C1 (♀) and normal Kunming mouse. Lanes 1-8, genomic DNA from F1 offspring derived from mating mentioned above. Lanes 3, 4 and 7 demonstrated the 767-bp specific band amplified from genomic DNA of F1 offspring. Thus these data demonstrated that founder C1 would transmit the foreign transgene to subsequent generation.
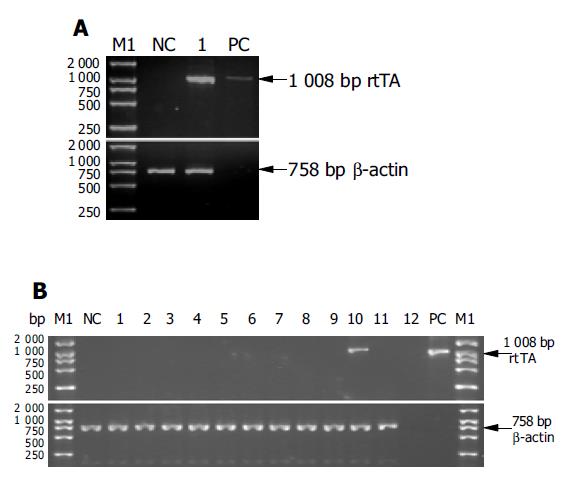
The regulatory sequence of the human apoE gene was used to achieve liver-specific expression of rtTA in one set of transgenic mice. RT-PCR of whole liver RNA was used to evaluate rtTA gene expression in the transgenic mice. rtTA mRNA was readily detected in total liver RNA from one F1 transgenic (+) animal derived from founder C1 (Figure 4A), and not detected in liver RNA from non-transgenic (-) littermate control (Figure 4A) and normal animal control (data not shown). A typical analysis of the PCR products by agarose gel electrophoresis from a positive-control ApoE-rtTA plasmid DNA (lane PC), RNA from one transgenic F1 offspring from founder C1 (lane 1), and RNA from a non-transgenic littermate (lane NC) is shown, while the result from RNA of a normal Kunming mouse is not indicated. β-actin served as an internal control to check the integrity of each tissue RNA sample and normalize for the quantity of input total RNA. Data were representative of three independent RT-PCRs that yielded similar results. Female offspring (F1) of founder C1 was surveyed for transgene (rtTA) expression in the different tissues by RT-PCR. A typical analysis of the RT-PCR products by agarose gel electrophoresis from RNA of brain (lane 1), heart (lane 2), lung (lane 3), kidney (lane 4), spleen (lane 5), muscle (lane 6), intestine (lane 7), stomach (lane 8), eye (lane 9), liver (lane 10), gonad (lane 11) and a non-transgenic littermates (lane NC) is shown. Results from reagent control (lane 12) and a positive-control ApoE-rtTA plasmid DNA (lane PC) are also indicated, while the result from RNA of a normal Kunming mouse is not shown. Furthermore, the transgenic animals were phenotypically similar to their non-transgenic (-) littermates and normal animals, and did not exhibit a detectable histologic change in the liver (data not shown). The results apparently suggested that rtTA gene integrated into the genome of one line from founder C1 could be normally expressed in the liver of transgenic mice.
To confirm the tissue-specificity of transgene expression, an initial survey of transgene expression in a variety of tissues was performed on one mouse line from C1 by RT-PCR. RNA isolated from brain, heart, lung, kidney, spleen, muscle, intestine, stomach, eye and gonad of a female progeny (F1) from C1 did not demonstrate rtTA expression by RT-PCR, suggesting that the transgene expression was tightly restricted to the liver (Figure 4B).
Together, these findings demonstrate that apoE promoter appropriately drives rtTA expression in the murine liver.
Recent advances in molecular biology have enabled examination of the function of genes of interest by raising stable cell lines or transgenic animals with consistent gene expression[16-18]. However, if the transgene is harmful or disadvantageous for cell growth or embryogenesis, the resultant cell lines or animals may be already genetically changed to tolerate the effects of the transgene products because the transgene behaves as a self antigen, inducing negative selection of reactive T cells in the thymus[16-18]. Therefore, the immunologic response to the transgene cannot be easily studied without special manipulation. This is particularly obvious in models of autoimmune or viral disease. To circumvent these problems, it is necessary to develop a system by which the expression of a transgene can be induced at desired time points and otherwise be kept completely silent for an extended period of time. Such a model may also allow a viral or self-antigen to escape the thymic selection process so the immunologic response can be studied. Using conditional gene expression technology, it is possible to override such restrictions mentioned above to achieve temporal and tissue-specific manipulation of gene expression in vivo. Conditional gene expression in vivo has been achieved using a variety of model systems[16-18]. One of them takes advantage of the Cre/lox P recombination system by which a transgene can be activated in a tissue-specific and time-dependent manner; however, this system requires the exogenous delivery of Cre gene (usually by Cre transgenic mice or an adeno- or retrovirus), and the induction is irreversible, that is to say, these strategies of transgene regulation can only be carried out once, i.e., ‘off to on’ or ‘on to off’, when manipulated by Cre alone; however, sequential gene regulation such as a ‘off-on-off’ or ‘on-off-on’ strategy may be a valuable tool, especially in time-course experiments, to obtain further functional information [16-18,27,28]. The Tet-on/Tet-off expression systems, which are the most widely used inducible regulation system as a reliable excellent tool for stringent reversible, temporal and spatial control of transgene expression, are another choice to overcome the limitation(s) in vivo[19,20].
The availability of the Tet-on/Tet-off systems raises questions concerning under which conditions one should be preferred over the other. Based on the characteristics of two systems, the Tet-on system seems more appropriate for this project if a transgene is to be kept inactive most of the time and turned on only occasionally[19,20]. Though this system and other rtTA-based systems face the disadvantage, i.e., basal transgene leak in vivo, several approaches have been developed to effectively avoid this limitation[21,29].
In this study, the liver-specific promoter systems were employed to drive the viral proteins expression at the physiologically relevant site of hepatocytes. Presently the liver-specific promoters mainly include human serum albumin (Alb) promoter[9,30], MUP promoter (mouse major urinary protein promoter)[9,12], hSAP promoter (human serum amyloid P component promoter)[31], and apoE promoter (human apolipoprotein E promoter)[22,32-34]. In our laboratory, the vector of pLiv.7, containing apoE promoter, is used as the basic vector backbone to prepare the transgenic construct. Actually, when the target gene(s) is/are placed downstream of the human apoE promoter/intron for a liver-specific expression manner of gene, and upstream of the apoE polyadenylation sequences/liver element, which has been shown to ensure the high expression of target gene (Figure 1)[22,32-34], high-level expression of target gene(s) in transgenic mouse liver without interfering with mouse development has been often seen[22,32-34]. These points on the characteristics of the pLiv.7 and apoE promoter were also verified by the present study.
In Tet-on regulated transgenic mice, tissue specificity of target gene expression was conferred by the promoter driving rtTA expression[16-18]. Thus, rtTA expression in transgenic mouse liver was controlled by apoE promoter, which in turn determined the site(s) of HBV and/or HCV transgene expression in double-transgenic mice.
Studies on the fate of foreign DNA introduced into an established mammalian genome are of considerably general interest. In almost all instances, transgenic DNA is stably maintained once it has integrated into the genome; even when there are multiple copies if DNA integrated in a tandem head-to-tail array, the transgenic DNA is transmitted stably from one generation to the next without genomic rearrangements and without deletions[35,36]. However, examples of transgenic families with genetic instability under selective pressure have been identified[37]. The tyrosinase transgenic families have all displayed stable Southern hybridization patterns, and those families with stable pigmentation have maintained a uniform pigmentation intensity over more than 10 generations of mating[37]. Because alterations of the integration site would be expected to change both tyrosinase expression and pigmentation, these results imply that genetic instability is very rare for these transgenic inserts[37]. In the rtTA transgenic mice, the rtTA transgene have been already stably carried over from founder to its progeny (F1).
In summary, in the present study, we successfully generate the effector transgenic mice, e.g., ApoE-rtTA transgenic mice, in which rtTA expression can be tightly targeted at the murine liver.
We express our deepest gratitude to Dr. C.Y. Fan (Department of Pathology and Otolaryngology, University of Arkansas for Medical Sciences, USA) for his unstinting advice and technical guidance in making transgenic mice, for his supportive and friendly attitude toward this project, and for the reagents. We are also indebted to the expert technical assistance of JY Han, HH Zhang, GG Qiu, Y Ma, YL Lin, WG Huang, FY Chen, FR Ni, JY Xie and JH Wang at Center of Experimental Animal, Zhongshan University, and H Tang at the Third Military Medical University, Chongqing, China.
Co-correspondents: Dong Xiao
| 1. | Moradpour D, Cerny A, Heim MH, Blum HE. Hepatitis C: an update. Swiss Med Wkly. 2001;131:291-298. [PubMed] |
| 2. | Rosenberg S. Recent advances in the molecular biology of hepatitis C virus. J Mol Biol. 2001;313:451-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 151] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Gordon JW, Scangos GA, Plotkin DJ, Barbosa JA, Ruddle FH. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci USA. 1980;77:7380-7384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 744] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 4. | Akbar SK, Onji M. Hepatitis B virus (HBV)-transgenic mice as an investigative tool to study immunopathology during HBV infection. Int J Exp Pathol. 1998;79:279-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Feitelson MA, Larkin JD. New animal models of hepatitis B and C. ILAR J. 2001;42:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Koike K. Hepatocarcinogenesis in hepatitis viral infection: lessons from transgenic mouse studies. J Gastroenterol. 2002;37 Suppl 13:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Milich DR. Transgenic technology and the study of hepatitis viruses: a review of what we have learned. Can J Gastroenterol. 2000;14:781-787. [PubMed] |
| 8. | Fausto N. A mouse model for hepatitis C virus infection? Nat Med. 2001;7:890-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Kawamura T, Furusaka A, Koziel MJ, Chung RT, Wang TC, Schmidt EV, Liang TJ. Transgenic expression of hepatitis C virus structural proteins in the mouse. Hepatology. 1997;25:1014-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 912] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 11. | Moriya K, Yotsuyanagi H, Shintani Y, Fujie H, Ishibashi K, Matsuura Y, Miyamura T, Koike K. Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J Gen Virol. 1997;78:1527-1531. [PubMed] |
| 12. | Pasquinelli C, Shoenberger JM, Chung J, Chang KM, Guidotti LG, Selby M, Berger K, Lesniewski R, Houghton M, Chisari FV. Hepatitis C virus core and E2 protein expression in transgenic mice. Hepatology. 1997;25:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Soguero C, Joo M, Chianese-Bullock KA, Nguyen DT, Tung K, Hahn YS. Hepatitis C virus core protein leads to immune suppression and liver damage in a transgenic murine model. J Virol. 2002;76:9345-9354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Wakita T, Taya C, Katsume A, Kato J, Yonekawa H, Kanegae Y, Saito I, Hayashi Y, Koike M, Kohara M. Efficient conditional transgene expression in hepatitis C virus cDNA transgenic mice mediated by the Cre/loxP system. J Biol Chem. 1998;273:9001-9006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Wakita T, Katsume A, Kato J, Taya C, Yonekawa H, Kanegae Y, Saito I, Hayashi Y, Koike M, Miyamoto M. Possible role of cytotoxic T cells in acute liver injury in hepatitis C virus cDNA transgenic mice mediated by Cre/loxP system. J Med Virol. 2000;62:308-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Lewandoski M. Conditional control of gene expression in the mouse. Nat Rev Genet. 2001;2:743-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 546] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 17. | Ryding AD, Sharp MG, Mullins JJ. Conditional transgenic technologies. J Endocrinol. 2001;171:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | van der Weyden L, Adams DJ, Bradley A. Tools for targeted manipulation of the mouse genome. Physiol Genomics. 2002;11:133-164. [PubMed] |
| 19. | Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547-5551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3634] [Cited by in RCA: 3798] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 20. | Gossen M, Freundlieb S, Bender G, Müller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1863] [Cited by in RCA: 1854] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 21. | Zhu Z, Zheng T, Lee CG, Homer RJ, Elias JA. Tetracycline-controlled transcriptional regulation systems: advances and application in transgenic animal modeling. Semin Cell Dev Biol. 2002;13:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Allan CM, Taylor S, Taylor JM. Two hepatic enhancers, HCR.1 and HCR.2, coordinate the liver expression of the entire human apolipoprotein E/C-I/C-IV/C-II gene cluster. J Biol Chem. 1997;272:29113-29119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: A Laboratory Manual. 3rded. New York: Cold Spring Harbor Press 2003; 1-600. |
| 24. | Sambrook JE, Fritsch F, Maniatis T. Molecular Cloning: A Laboratory Manual. 3rded. New York: Cold Spring Harbor Laboratory Press 2001; 1-800. |
| 25. | Andre-Garnier E, Robillard N, Costa-Mattioli M, Besse B, Billaudel S, Imbert-Marcille BM. A one-step RT-PCR and a flow cytometry method as two specific tools for direct evaluation of human herpesvirus-6 replication. J Virol Methods. 2003;108:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Mizutani T, Nishino Y, Kariwa H, Takashima I. Reverse transcription-nested polymerase chain reaction for detecting p40 RNA of Borna disease virus, without risk of plasmid contamination. J Vet Med Sci. 1999;61:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000;26:99-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 28. | Sauer B. Inducible gene targeting in mice using the Cre/lox system. Methods. 1998;14:381-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 510] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 29. | Zhu Z, Ma B, Homer RJ, Zheng T, Elias JA. Use of the tetracycline-controlled transcriptional silencer (tTS) to eliminate transgene leak in inducible overexpression transgenic mice. J Biol Chem. 2001;276:25222-25229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Kato T, Ahmed M, Yamamoto T, Takahashi H, Oohara M, Ikeda T, Aida Y, Katsuki M, Arakawa Y, Shikata T. Inactivation of hepatitis C virus cDNA transgene by hypermethylation in transgenic mice. Arch Virol. 1996;141:951-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Matsuda J, Suzuki M, Nozaki C, Shinya N, Tashiro K, Mizuno K, Uchinuno Y, Yamamura K. Transgenic mouse expressing a full-length hepatitis C virus cDNA. Jpn J Cancer Res. 1998;89:150-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Fan J, Wang J, Bensadoun A, Lauer SJ, Dang Q, Mahley RW, Taylor JM. Overexpression of hepatic lipase in transgenic rabbits leads to a marked reduction of plasma high density lipoproteins and intermediate density lipoproteins. Proc Natl Acad Sci USA. 1994;91:8724-8728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 195] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Majumder M, Ghosh AK, Steele R, Zhou XY, Phillips NJ, Ray R, Ray RB. Hepatitis C virus NS5A protein impairs TNF-mediated hepatic apoptosis, but not by an anti-FAS antibody, in transgenic mice. Virology. 2002;294:94-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Yamanaka S, Balestra ME, Ferrell LD, Fan J, Arnold KS, Taylor S, Taylor JM, Innerarity TL. Apolipoprotein B mRNA-editing protein induces hepatocellular carcinoma and dysplasia in transgenic animals. Proc Natl Acad Sci USA. 1995;92:8483-8487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 230] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 35. | Jackson IJ, Abbott CM. Mouse Genetics and Transgenics: A Practical Approach. London: Oxford University Press 2000; 1-350. |
| 36. | Tymms MJ, Kola I. Gene knockout protocols. Totowa: Humana Press Inc 2001; 1-370. |
| 37. | Overbeek PA. Factors affecting transgenic animal production. Transgenic Animal Technology: A Laboratory Handbook. San Diego: Academic Press Inc 1994; 69-114. |