INTRODUCTION
Esophageal squamous cell carcinoma (ESCC) is one of the most common fatal cancers worldwide, and the highest rates of esophageal cancer in the world occur in north-central China. Although tremendous advances in diagnosis and treatment have been achieved recently, esophageal cancer is still one of the most lethal malignancies mainly because of its later discovery. However, many cases of esophageal carcinoma could be cured and even prevented if there were better screening methods to uncover the disease when it is limited and most responsive to intervention. A possibility is to screen patients for the expression of various regulatory genes. The etiology of esophageal cancer has been partially elucidated using recently devised molecular biological techniques. The homeobox (Hox) genes are a family of regulatory genes that contain a common 183-nucleotide sequence and code for specific nuclear proteins (homeoproteins) that act as transcription factors. In addition to their roles in axial patterning during embryonic development[1-4], Hox genes help control the normal cellular proliferation and differentiation of several adult tissues[5-7] and the proliferation and oncogenic transformation in some neoplasms and cancers[8-11].
HoxD9 is the member of Abd-B related HoxD genes located closest to the 3’ end of the chromosome. It is involved in the development and patterning of the forelimb and axial skeleton[12]. HoxD9 gene is also known to be a potential transcription factor that not only autoregulates itself but also transactivates other Hox genes and certain members of other gene families[13,14].
The Pbx1 proto-oncogene, another transcription factor, was originally identified at the site of t(1;19) chromosomal translocations in acute pre-B-cell leukemia. The Pbx1 gene codes for two isoforms of the homeodomain (HD) DNA-binding motif[15,16]. Human pre-B-cell acute leukemias are frequently associated with t(1:19)(q23; p13,3) chromosomal rearrangement, which creates a chimeric gene encoding a fused E2a and Pbx1 proteins[17-20]. Aberrant E2a transcripts lacking the helix-loop-helix DNA-binding motif have been detected in several stable cell lines carrying the translocation[16]. Fusion cDNAs have been shown to encode an 85 KDa protein composed of the N-terminal two-thirds transactivation domain of the E2a protein fused to a homeoprotein termed Pbx1[16-18]. Thus Pbx1 is a class II Hox gene. Although homeoprotein can bind to DNA as monomers, dimerization with Pbx homeoproteins substantially increases the DNA-binding activity of these transcription factors[21].
We examined expression pattern of two novel oncofetal antigens, the HoxD9 and Pbx1 homeoproteins in ESCC by using a sensitive immunocytochemical method to evaluate if they could be a screening tool for esophageal cancer.
MATERIALS AND METHODS
Tissue handling and storage
All 56 ESCC from patients whose tissues used in the retrospective study were formalin-fixed, paraffin-embedded archival specimens obtained from the pathology tissue banks. The patients underwent esophagectomy at Peking University School of Oncology, Beijing Cancer Hospital without preoperative chemotherapy or radiotherapy. These tissues were fixed in 10% formalin buffered with phosphate to pH 7.4 and were then embedded in paraffin. The diagnosis of ESCC was established and confirmed by staff pathologists in the Department of Pathology of Beijing Cancer Hospital. This study was approved by the Institutional Board for Clinical Research.
Antibodies
Anti-HoxD9 antibody (H-342; Santa Cruz Biotechnology, Santa Cruz, CA) is a rabbit polyclonal antibody raised against a recombinant protein corresponding to amino acids 1-342, a sequence that represents full-length HoxD9 of human origin. This antibody is recommended by the company for detection of HoxD9, HoxC9, HoxA9 and HoxB9 of mouse, rat, and human origin by Western blotting, immuno-precipitation, and immunohistochemistry. Anti-Pbx1 antibody (P-20; Santa Cruz Biotechnology) is an affinity-purified rabbit polyclonal antibody raised against a peptide mapped to the amino terminus of Pbx1 of human origin. This antibody reacts with Pbx1 of mouse, rat, and human origin as detected by Western blotting and immunohistochemistry, and it does not cross-react with Pbx2 or Pbx3.
Immunocytochemical antigen-detection technique
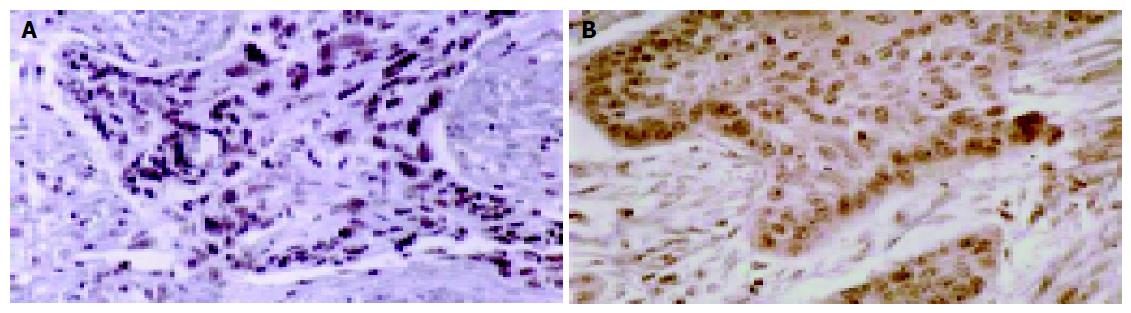
We used a highly sensitive, indirect four-step immunocy-tochemical method[22-26] in formalin-fixed, paraffin-embedded ESCC tissue samples to detect HoxD9 and Pbx1 proteins. Briefly, the samples were deparaffinized by three changes of xylene substitute for 20-30 min, and then rehydrated using decreasing dilutions of alcohol (100%, 95%, 85%, 70%, and 50% to PBS). An initial blocking step using 1% hydrogen peroxide was necessary to eliminate endogenous alkaline phosphatase activity. A second blocking step was conducted with purified goat serum for antigenic epitopes and excess serum was removed from the area surrounding the sections. The tissue sections were incubated with primary anti-HoxD9 or anti-Pbx1 antibodies for 90-120 min, and then with the secondary antibody for 20 min. Immunostaining of the antigen primary antibody complex was performed using the avidin-biotin-peroxidase complex with the LSAB kit, peroxidase (Dako, Kyoto, Japan) to allow formation of a stable yellow-brown precipitate and was followed by light hematoxylin counterstaining. Finally, short- and long-term morphologic clearings were carried out with two changes of xylene substitute.
As a control, some sections of each tissue sample were not incubated with primary antibody. Other sections were not treated with hydrogen peroxide so we could confirm the presence of endogenous alkaline phosphatase activity.
Tissue evaluation
Qualitative and quantitative evaluation of the percentage of antigen-positive cells as determined by their yellow-brown color and intensity of staining were conducted using a light microscope (Olympus, Kyoto, Japan). For each section, we counted 100-200 cells each from five distinct areas in non-necrotic, non-hemorrhagic ESCC tissues. Artifacts were avoided, and areas with morphologic characteristics of interest were identified. The intensity of staining was characterized as very intense, strong, light, or negative[22]. We calculated the percentage of cells that exhibited any staining[22]. (++++) indicates that >90% of the total cell number are positive; (+++) indicates that 51-90% of the total cell number are positive; (++) indicates 11-50% of the total cell number are positive; (+) indicates 1-10% of the total cell number are positive; (–) are negative.
DISCUSSION
The presence of HoxD9 and Pbx1 demonstrated the re-expression of these transcriptional regulators of oncogenesis and histogenesis after neoplastic transformation of esophageal epithelium and its cellular differentiation. Bodey and his colleagues established the expression pattern of three homeoproteins (Hox-B3, -B4, and -C6) in human lung carcinoma[23], osteosarcoma[24], breast carcinoma[25], and childhood medulloblastoma[26] tissues. Using the same immunocytochemical technique we did two Hox gene products in human ESCC cells. Thus re-expression of Hox gene products occurs in a wide variety of neoplastically transformed cells, and homeoproteins appear to be a family of oncofetal antigens involved in both normal cellular development and in cellular carcinogenesis and tumor progression.
Genes that have been shown to be controlled by homeoproteins encode adhesion molecules, transcription factors (in particular the Hox genes themselves), and growth factors. At present, the putative targets of HoxD9 include other members of the Hox family and adhesion molecules, such as the liver cell adhesion molecule[13,14]. At least one Hox gene, HoxB7, has been shown to activate basic fibroblast growth factor (bFGF) transcription in melanomas by binding to the promoter of bFGF gene[27]. Moreover, transduction of the breast carcinoma cell line with the HoxB7 gene induces bFGF expression and increases cell proliferation of teratocarcinoma cells[28]. Whether HoxD9 directly affects the regulation of bFGF and c-Fos, important factors for cell proliferation and transformation is uncertain and needs further investigation. However, bFGF and c-Fos were found to be up-regulated in HoxD9 transfected synoviocytes. HoxD9 appears to play a role in bFGF-induced proliferation, but not so much so in the tumor necrosis factor pathway[29].
Pbx proteins comprise a functionally and biochemically distinct subclass of homeoproteins. A similar role for Pbx is indicated by this protein’s potential involvement in a highly conserved autoregulatory loop that controls HoxB1 expression in the mouse hindbrain[30]. Pbx1 was discovered as a fusion with the E2a gene after chromosomal translocations in a subset of acute leukemias. The resulting E2a-Pbx1 chimeric proteins display potent oncogenic properties that appear to require dimerization with Hox DNA-binding partners. E2a-Pbx1 heterodimerizes with Hox but not with MEIS proteins (members of the TALE family of homeoproteins), produces acute myeloid leukemia in mice, and blocks differentiation of cultured murine myeloid progenitors. Calvo et al[31] reported that a 25-residue predicted alpha-helix preceding the Pbx1 HD bound this HD and prevented both its binding to DNA and its ability to heterodimerize with Hox proteins. Addition of 39 residues at the N terminus to this inhibitory helix revealed a Pbx dimerization interface that orchestrated cooperative DNA-binding of E2a-Pbx1 and exposed all Pbx proteins as homodimers and heterodimers. Sequences inhibiting DNA-binding and mediating Pbx dimerization coincided with those reported to have nuclear export function. An additional 103 residues at the N terminus side of the Pbx dimerization interface restored heterodimerization with Hox and MEIS1 proteins. This negative switch domain comprising of the inhibitory helix and N-terminal regions required for its partner-mediated derepression was dispensable to myeloid immortalization by E2a-Pbx1. Although the heterodimer was stabilized, the 310 helix C terminus to the Pbx1 HD was also dispensable to E2a-Pbx1’s ability to heterodimerize with Hox proteins and immortalize myeloblasts. Retention of myeloid immortalization by E2a-Pbx1 proteins lacking all Pbx1 sequences for the N or C terminus to the HD indicates that Hox proteins cooperate with E2a-Pbx1 in myeloid immortalization[31].
The DNA-binding affinity and specificity of homeoproteins is augmented by cofactor interactions. Hox cofactors in mammals include Pbx[32] and MEIS[33]. Pbx proteins bind DNA cooperatively as heterodimers with MEIS family members and also with homeoproteins from paralog groups 1 to 10. MEIS proteins cooperatively bind DNA with the Abd-B class of homeoproteins groups 9 and 10. The most important aspects of this binding are that most of the Pbx N terminus to the HD is required for efficient cooperative binding with HoxD4 and HoxD9; MEIS and Pbx proteins form higher-order complexes on a heterodimeric binding site; MEIS forms a similar trimer with DNA-bound Pbx-HoxD9; and an additional trimer class involving non-DNA-bound Pbx and DNA-bound MEIS-HoxD9 or MEIS-HoxD10 heterodimers is enhanced by mutation of the Pbx HD[34]. These findings suggest novel functions for Pbx and MEIS in modulating the function of DNA-bound MEIS-Hox and Pbx-Hox heterodimers, respectively.
Retinoic acid induces expression of genes encoding the Hox family of transcription factors, whose differential expression orchestrates developmental programs specifying anterior-posterior structures during embryogenesis, thereby possibly inducing various effects on Hox gene expression based on the subset organization of its receptors[35]. Homeoproteins bind DNA as monomers and Pbx proteins as heterodimers. Retinoic acid up-regulated Pbx expression coincident with transcriptional activation of Hox genes in P19 embryonal carcinoma cells undergoing neuronal differentiation. However, in contrast to Hox induction, Pbx up-regulation was predominantly a result of post-transcriptional mechanisms. Pbx1, as well as its highly related family members Pbx2 and Pbx3, exhibited different profiles of up-regulation, suggesting possible functional divergence[35]. The parallel up-regulation of Pbx and Hox proteins in this model suggests an important role for transcriptional control by Pbx-Hox heterodimers during neurogenesis and provide evidence of precise control by retinoic acid[36].
The function of homeoproteins may be modulated by various secreted factors, such as growth factors, cytokines, and hormones. The involvement of HoxD9 in the regulation of cellular growth might be mediated, at least in part, by up-regulation of growth factors such as bFGF and c-Fos or might result from increased transcription activity by its regulators[34]. The expression of HoxC6 in osteosarcomas and neuroblastomas is differentially regulated by rhBMP-2, tumor growth factor-beta, and activin-A, which suggests that specific Hox genes may be target genes for tumor growth factor-beta superfamily members and may represent a way in which growth factors exert their immense effects on development and carcinogenesis[37].
Many adjuvant therapies and surgical procedures have been examined to improve survival among patients with esophageal cancer. Surgical resection rates have improved strikingly, and operative mortality has decreased markedly. However, the curative potential of surgery is likely highest when the disease is detected while it is still in early stage. Our data suggest that two Hox genes, HoxD9 and Pbx1, are inappropriately expressed in most ESCCs. The exact mechanisms that regulate the re-expression of these oncofetal antigens in ESCC, as well as other solid tumors, should be elucidated through further basic research. Understanding the role of Hox genes in esophageal epithelial cell carcinogenesis may not only increase early detection but also offer new avenues of treatment for this disease.