Published online Mar 15, 2004. doi: 10.3748/wjg.v10.i6.903
Revised: April 23, 2003
Accepted: May 21, 2003
Published online: March 15, 2004
AIM: To detect the origin of hepatocellular carcinoma (HCC) recurring and attempt to propose a new recurrent mechanism.
METHODS: Orthotopic liver allotransplantation was performed on male rats with HCC- induced by diethylnitrosamine using female donors. Metastatic tumors in transplanted livers were obtained. A DNA probe that exhibits specificity for the rat Y chromosome was generated by using a set of primers specific to murine sry gene. In situ hybridization (ISH) for Y chromosome was used to detected the origin of HCC recurring. Male HCC tissue was designed to be positive control. ISH on female tissue and using non-labeled with DIG probe was thought to be negative control.
RESULTS: Positive marks were seen through ISH for Y chromosome in recurrent tumor tissue and positive control. No signal was detected in both negative controls.
CONCLUSION: Recurrent HCC after liver transplantation originated from disseminated tumor cells in recipients. Extrahepatic cells homing into liver may be a new HCC recurrence mechanism. Likewise, it implicates that this mechanism is responsible for HCC recurring after hepatectomy.
- Citation: Li QG, Yang GS, Yang Q, Wei LX, Yang N, Zhou XP, Jia FQ. Disseminated tumor cells homing into rats’ liver: A new possible mechanism of HCC recurrence. World J Gastroenterol 2004; 10(6): 903-905
- URL: https://www.wjgnet.com/1007-9327/full/v10/i6/903.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i6.903
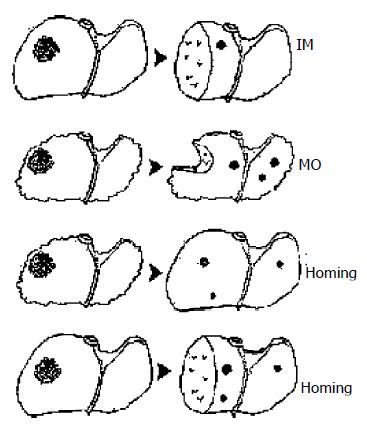
The poor outcomes of patients with hepatocellular carcinoma (HCC) are mainly resulted from high postoperative recurrence rate with 65% after radical resection versus 58% after liver transplantation[1,2]. Over the past decades, many investigations using clinicopathological and molecular biological methods have showed that there existed two HCC recurrent mechanisms of intrahepatic metastasis (IM) and multicentric occurrence (MO)[3-5]. However, the both mechanisms are very reluctant to elucidate the HCC recurrence following liver transplantation in rigidly selected patients. Therefore, we refer a hypothesis that recurrent HCC in transplanted liver is likely to originate from extrahepatic tumor cells in recipient if de novo carcinogenesis is excluded. Likewise, disseminated tumor cells possibly go back to remnant liver after hepatectomy.
We have established an animal model of liver transplantation for HCC in rats. Male rat liver with HCC induced by diethylnitrosamine (DENA) was replaced by allograft from normal syngenic female animal. Recurrent tumors from male recipient of female liver were analyzed using in situ hybridization (ISH) for the Y chromosome to indicate cells origins. On the basis of our investigation and other supportive literatures, we make an attempt to propose a new possible mechanism of HCC recurrence.
Ninety-eight inbred seven-week-old male SD rats, weight ranged from 100 g to 120 g, were purchased from PEAK Company in Shanghai with the approval of Shanghai Animal Committee. HCC was induced by oral administrantion of 100 ppm DENA (Sigma Company, USA) water solution. OLT for HCC was performed according to Kamada cuff techniques[6] on male rats with HCC and donor livers were from normal syngenic female SD rats with weight ranged from 250 g to 300 g. No immunosuppressant was postoperatively administered. Explanted livers were examined pathologically. The recurrent tumors in the transplants may be explored through laparatomy before the recipients’ death. Harvested specimens were preserved at -70 °C.
Male rat genomic DNA was purified from 300 μL blood sample using Wizard Genomic DNA Purification Kit (Promega). sry gene specific primer, of which the sequences are 5’- CAGAGATCAGCAAGCAGCTG-3’ and 5’-TGCAGCTCTACTCCAGTCTTG-3’, was synthesized by Shenggong Biochemical Incorporation in Shanghai. 0.1 μg of the genomic DNA as template, sry gene was amplified by PCR. The reaction was comprised of 35 cycles of 5 min at 94 °C, 0.5 min at 60 °C, 1 min at 72 °C. PCR products were analyzed on 15 g/L agarose gel and then purified with QIAquick Gel Extraction Kit (Qiagen). Target gene was labeled according to the instruction of DIG High Prime DNA Labeling and Detection Starter Kit II (Roche).
Male SD rat HCC tissue confirmed by pathology was designed to be positive control. ISH on female SD rat liver tissue and using non-labeled with DIG probe was thought to be negative control. ISH efficacy would be verified by the both controls.
Frozen tissue sections (5 μm in thickness) were fixed on the slides treated by 40g/L polyformaldehyde. Slides were washed in PBS (PH7.4) for 5 min (2 times), 3g/L Triton X-100/PBS for 10 min, and PBS (PH7.4) for 5 min (2 times). Tissue was digested with pepsin 2 μg/mL for 15 min at 37 °C, and then washed in PBS for 5 min and rinsed in 4 × sodium saline citrate (SSC) for 2 min at room temperature. Slides were denatured with 50% formamide in 2 × SSC for 15 min at room temperature, then dehydrated and air dried. Probe for Y chromosome (10 ng/μL) was denatured for 5 min at 75 °C, added to the denatured tissue, coverslipped and incubated in a humid chamber overnight at 42 °C. Slides were then washed in 2 × SSC for 10 min (2 times), 1 × SSC for 10 for min (2 times), 0.1 × SSC 10 for min (3 times), and in buffer1 (Tris-HCl, PH7.5, 100 mmol/L; NaCl 150 mmol/L) for 5 min. Anti-DiG-Ap (1:500 dilution) was added to tissues and incubated for 2 h at 43 °C. Slides were washed in buffer1 for 5 min (2 times) and buffer 2 (Tris-HCl, PH9.5, 100 mmol/L; NaCl 100 mmol/L, MgCl2 50 mmol/L) for 10 min. And then slides were transferred to NBT/BCIP solution to be stained for more than 2 h, and rinsed with distilled water. Tissue sections were mounted and evaluated by light microscopy.
HCC recurrence was found in 6 transplants from 98 transplanted rats. Of them, 3 grafts were discarded because the recipients have been dead when laparotomy. Thirteen lesions were obtained in other three available transplants, confirmed to HCC by pathological examination. These specimens were preserved at -70 °C as a bank for further utilization.
HCC cells carrying a positive reaction product (blue staining) were seen in control male rats. No signal was detected in the controls of female liver and system using non-labeled probe. Control trials showed that the sry gene specific probe was efficient.
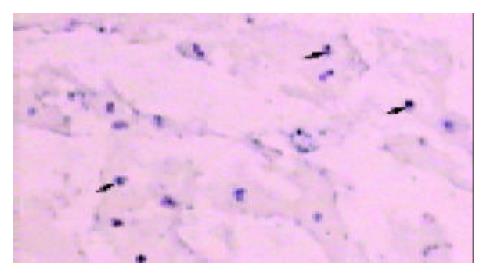
Positive staining was seen in frozen sections of the 3 recurrent transplants. Typically, the probe was successfully hybridized with target gene on tumor cells, whereas failed to on para-tumor tissues. The capsule, aimed at by arrow, definitely parts the recurrent tumor from the recipient liver parenchyma under 10 × 10 magnification (Figure 1).
Liver transplantation for HCC in rats provides an excellent animal model to carcinogenesis investigation[9]. Intrahepatic tumors and underlying lesions are removed completely, and then some interventional trials could be executed etiologically. In our experiments, no immunosuppressants were administered because rejective reaction was weak in allotransplantation on syngenic SD rats. The promotion of immunosuppressants to tumor growth can be excluded. However, it remains indefinite that induction of HCC existed in recipients after the withdrawal of DENA[10]. There were two possible mechanisms of tumor recurring with the inclusion of disseminated tumor cells homing into implanted liver and sequential contribution of DENA. Therefore, a marker is a key to discriminate between the two possibilities.
Cellular markers have been obtained through varieties of strategies such as transgenic animal, retroviral transduction. But these strategies are very complicated and low efficient. Discordant gender transplants offer the benefit of having 100% of the cells marked, as opposed to retroviral transduction, where at best only 5-10% of the cells are marked[11]. In our experience, Y chromosome, uniquely existed in male cell, was initially acted as a marker with which male and female tissues were differentiated. Y chromosome-specific probe was successfully hybridized with sry gene of which multiple copies facilitated this hybridization[7]. This in situ hybridization system was proved to be reliable by positive and negative control we designed. Our results therefore indicated that the origin of recurrent HCC in female liver was from disseminated tumor cells in male recipient. We personally define this phenomenon that extrahepatic tumor cells go back to liver as “homing”.
Other studies could support the homing hypothesis we proposed. (1). Alpha-fetoprotein (AFP) messenger RNA (mRNA) has been proposed as a marker of HCC cells disseminated into the circulation. Multiple molecular methods, such as nested and semi-quantitative retro-transcription polymerase chains reaction (RT-PCR), have been utilized to detect AFP mRNA in order to confirm the presence of hematogenous HCC cells. This marker expression in peripheral blood of patients with HCC indicates the existence of tumor cells, although it remains controversial that AFP mRNA is taken as an evidence of HCC recurrence[12-14]. (2). The homing of both lymphocytes and malignant hematic cells has been acknowledged[15,16]. Involved adhesion molecules that mediate their migration also contribute to invasiveness of liver cancer[16]. (3). Metastasis is the result of multiple sequential steps and is a highly organized, nonrandom, and organ-selective process[17]. A group of biological molecules is collectively responsible for determining whether tumor cells can progress from a single malignant cell to a metastatic disease[17]. But metastatic cells eventually colonize a particular organ that provides an optimal microenvironment[18,19]. It therefore is warranted that transplanted liver or regenerating liver after resection may be a particularly fertile ground for extrahepatic HCC cells to proliferate.
On the basis of this experiment, we concluded that recurrent HCC after liver transplantation originated from the disseminated tumor cells. These extrahepatic cells homing into liver may be a new HCC recurrence mechanism. Likewise, it implicates that this new mechanism is responsible for HCC recurrence after hepatectomy besides MO and IM (Figure 2). But our experiment, to some extent, was to describe a homing phenomenon. Its real mechanism needs to be further investigated by molecular biology.
Edited by Ma JY and Xu FM
| 1. | Schlitt HJ, Neipp M, Weimann A, Oldhafer KJ, Schmoll E, Boeker K, Nashan B, Kubicka S, Maschek H, Tusch G. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg. 2000;191:381-388. [RCA] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 177] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Schlitt HJ, Neipp M, Weimann A, Oldhafer KJ, Schmoll E, Boeker K, Nashan B, Kubicka S, Maschek H, Tusch G. Recurrence patterns of hepatocellular and fibrolamellar carcinoma after liver transplantation. J Clin Oncol. 1999;17:324-331. [PubMed] |
| 3. | Nakashima O, Kojiro M. Recurrence of hepatocellular carcinoma: multicentric occurrence or intrahepatic metastasis A viewpoint in terms of pathology. J Hepatobiliary Pancreat Surg. 2001;8:404-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 5. | Kumada T, Nakano S, Takeda I, Sugiyama K, Osada T, Kiriyama S, Sone Y, Toyoda H, Shimada S, Takahashi M. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology. 1997;25:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 270] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Goto S, Kamada N, Delriviere L, Kobayashi E, Lord R, Ware F, Hara Y, Edwards-Smith C, Shimizu Y, Vari F. Orthotopic liver retransplantation in rats. Microsurgery. 1995;16:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | An J, Beauchemin N, Albanese J, Abney TO, Sullivan AK. Use of a rat cDNA probe specific for the Y chromosome to detect male-derived cells. J Androl. 1997;18:289-293. [PubMed] |
| 8. | Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 900] [Cited by in RCA: 851] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 9. | Schotman SN, Schraa EO, Marquet RL, Zondervan PE, Ijzermans JN. Hepatocellular carcinoma and liver transplantation: an animal model. Transpl Int. 1998;11 Suppl 1:S201-S205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Travis CC, McClain TW, Birkner PD. Diethylnitrosamine-induced hepatocarcinogenesis in rats: a theoretical study. Toxicol Appl Pharmacol. 1991;109:289-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Eckert JW, Buerkle CJ, Major AM, Finegold MJ, Brandt ML. In situ hybridization utilizing a Y chromosome DNA probe. Use as a cell marker for hepatocellular transplantation. Transplantation. 1995;59:109-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Gross-Goupil M, Saffroy R, Azoulay D, Precetti S, Emile JF, Delvart V, Tindilière F, Laurent A, Bellin MF, Bismuth H. Real-time quantification of AFP mRNA to assess hematogenous dissemination after transarterial chemoembolization of hepatocellular carcinoma. Ann Surg. 2003;238:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Ijichi M, Takayama T, Matsumura M, Shiratori Y, Omata M, Makuuchi M. alpha-Fetoprotein mRNA in the circulation as a predictor of postsurgical recurrence of hepatocellular carcinoma: a prospective study. Hepatology. 2002;35:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Wong IH, Lau WY, Leung T, Yeo W, Johnson PJ. Hematogenous dissemination of hepatocytes and tumor cells after surgical resection of hepatocellular carcinoma: a quantitative analysis. Clin Cancer Res. 1999;5:4021-4027. [PubMed] |
| 15. | Wiedle G, Dunon D, Imhof BA. Current concepts in lymphocyte homing and recirculation. Crit Rev Clin Lab Sci. 2001;38:1-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Aziz KA, Till KJ, Zuzel M, Cawley JC. Involvement of CD44-hyaluronan interaction in malignant cell homing and fibronectin synthesis in hairy cell leukemia. Blood. 2000;96:3161-3167. [PubMed] |
| 17. | Sun JJ, Zhou XD, Liu YK, Tang ZY, Feng JX, Zhou G, Xue Q, Chen J. Invasion and metastasis of liver cancer: expression of intercellular adhesion molecule 1. J Cancer Res Clin Oncol. 1999;125:28-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Ji XN, Ye SL, Li Y, Tian B, Chen J, Gao DM, Chen J, Bao WH, Liu YK, Tang ZY. Contributions of lung tissue extracts to invasion and migration of human hepatocellular carcinoma cells with various metastatic potentials. J Cancer Res Clin Oncol. 2003;129:556-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Fidler IJ. Seed and soil revisited: contribution of the organ microenvironment to cancer metastasis. Surg Oncol Clin N Am. 2001;10:257-269, vii-viiii. [PubMed] |