INTRODUCTION
Gastrointestinal dysfunction occurs frequently in patients with traumatic brain injury (TBI)[1-3]. More than 50% patients with severe head injuries do not tolerate enteral feedings[4]. This intolerance is manifested by vomiting, abdominal distention, delayed gastric emptying[5,6], esophageal reflux[7] and decreased intestinal peristalsis[8], indicating that gastrointestinal dysfunction is a common phenomenon following TBI. The association of severity of brain injury with the intolerance of enteral feeding suggests a strong link between the central nervous system and the nonfunctioning gut. However, the precise mechanism of gastrointestinal dysfunction following TBI has not been reported to date and remains to be interpreted.
Gastrointestinal motility is mainly regulated by two factors including humoral hormones and nervous transmitters from both central nervous system and peripheral enteric nervous system[9]. Brain-gut peptides possess two functions as mentioned above, e.g., exerting action on gastrointestinal motility via both endocrine hormones and peptidergic transmitters. These peptides also play their roles in modulation of the gastrointestinal motility through central nervous system, which is a part of brain-gut interaction[10]. Recent studies have indicated that some disorders of gastrointestinal motility following sepsis[11-13], trauma[14], surgery[15-17], chronic stress[18] and experimental spleen deficiency[19] are related to brain-gut peptides, such as cholecystokinin (CCK), vasoactive intestinal peptide (VIP), calcitonin gene-related peptide (CGRP), neuropeptide Y (NPY) and substance P (SP), suggesting that brain-gut peptides are important mediators in the regulation of gastrointestinal motility[20-22]. Until now, no study has been made to investigate the relationship between brain-gut peptides and gastrointestinal dysmotility following TBI. Therefore, we hypothesized that TBI could induce marked alterations of brain-gut peptides in both plasma and gut, which could be involved in the pathogenesis of complicated gastrointestinal dysfunction.
Brain-gut peptides as modulatory mediators appear to be major components of bodily integration and have important regulatory actions on physiological function of gastrointestinal tract. Increasing studies have demonstrated that VIP[14,17,19,23], CCK[12,20,21] and CGRP[11,15,16,24] play important protective role in regulation of blood flow, cell differentiation, immune function and secretion of digestive glands. On the other hand, they have an adverse effect on the gut motility[11-19]. It is also well known that VIP, CCK and CGRP are located in both central nervous system (CNS) and peripheral enteric nervous system, and mainly exert inhibitory action on gut motility[16-21,24]. Several kinds of stress, such as trauma, cold, restraint and pain, can induce the release of these brain-gut peptides from CNS and enteric nerve[14,18], and the alterations of relevant receptors in the gut[19] to induce the gastrointestinal dysmotility. However, it is unknown how these peptides change and what the underlying significance of these changes is in the gastrointestinal dysmotility following TBI. Further insights into the alterations of brain-gut peptides following TBI may provide new therapeutic opportunities for the gastrointestinal dysfunction complicating head trauma. In current study, rat models of TBI were made to measure the alterations of VIP, CCK and CGRP in both plasma and jejunum and to investigate the underlying role of brain-gut peptides in gastrointestinal dysfunction.
MATERIALS AND METHODS
Rat models of TBI
Male Wistar rats, weighing from 220 to 250 g, were purchased from Animal Center of Chinese Academy of Science, Shanghai, China. The rats were fed rodent chow with free access to tap water and housed in temperature- and humidity-controlled animal quarters with 12 h light/dark cycle. All procedures were approved by the Institutional Animal Care Committee.
The rats were randomly divided into six groups (6 rats/ group) including control group with right parietal bone window alone and no brain injury, and TBI groups at h 3, 12, 24 and 72, and day 7, respectively. Following intraperitoneal anesthesia with urethane (1 000 mg/kg), animal head was fixed in the stereotactic device. A right parietal bone window of 5 mm in diameter was made under aseptic conditions with dental drill just behind cranial coronal suture and beside midline. The dura was kept intact. Right parietal brain contusion was adopted from the impact method described by Feeney et al[25] and severe traumatic brain injury was made by dropping weight with impact diameter of 4 mm, depth of 5 mm and total impact energy of 1 000 g·cm. The control animals were killed for sample collection at 72 h, and TBI group rats were decapitated at corresponding time point. Blood samples were obtained by right ventricle puncture and centrifuged with the plasma stored at -40 °C for further analysis. A segment of the jejunum 15 cm distal to Treitz ligament was taken, rinsed and weighed, then were put into a tube with 1 mL saline water. The tube was plunged into vigorous boiling water and boiled for 3 min, and then all layers of jejunum sample and 0.5 mL of 1 mol/L glacial acetic acid were added to a homogenizer to be homogenized for 10 min. The 0.5 mL of 1 mol/L NaOH was mixed with the homogenized sample. After centrifugation at 3 000 r/min for 5 min at low temperature, the supernatant was collected and stored at -40 °C until assay of brain-gut peptides.
Assay analysis of VIP, CCK and CGRP in plasma
The concentrations of VIP, CCK and CGRP in plasma samples were measured by enzyme immunoassay (EIA) with commercially available kits according to the manufacturer’s instructions. Peptides [VIP (S-80039), CCK (S-80013-B), α-CGRP (S-80011-R)] were purchased from AOR Diagnostics, USA. The minimum detectable concentration for VIP, CCK and α-CGRP was 0.06-0.08 ng/L of sample with intra- and inter-assay variation less than 6% and 15% respectively. There was 25% cross-reaction with β-CGRP.
Assay analysis of VIP, CCK and CGRP in jejunum samples
VIP levels in jejunum were determined by radioimmunoassay (RIA) with the kit purchased from Beijing Huayin Biotechnology Institute, China. The concentrations of CCK and CGRP in all layers of jejunum were measured by EIA as mentioned above. The protein concentration of jejunum samples was determined by using a bicinchoninic acid assay kit with bovine serum albumin as the standard (Pierce Biochemicals, Rockford, IL).
Statistical analysis
Software SPSS 11.0 was used in the statistical analysis. Parameters were expressed as mean ± SD, and compared using one-way ANOVA analysis of variance, followed by Tukey test. Statistical significance was determined at P < 0.05.
RESULTS
Gross observations of gastrointestinal pathology
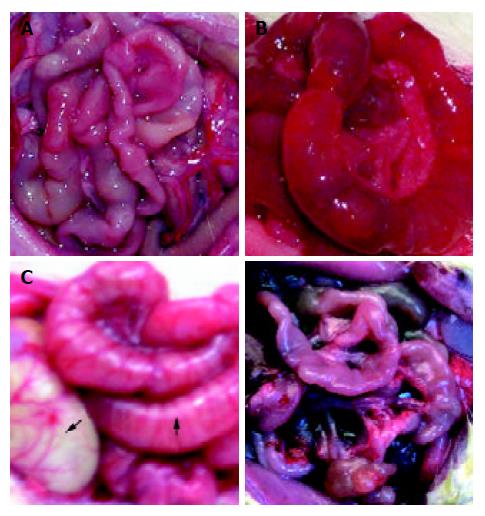
Gastrointestinal tract was found to be nearly normal in rats of control group and TBI 3 hour group (Figure 1A). In TBI groups at 12, 24 and 72 h postinjury, gastric distention and intestinal dilatation with a large amount of yellowish effusion existing within intestinal cavity were found, and intestinal wall became thinner and edematous (Figures 1B and 1C), suggesting that delayed gastric emptying and paralytic ileus occurred following TBI. The above mentioned pathological changes culminated at 72 h following TBI. On d 7 postinjury, small intestine was found to become thin and slender, suggesting that an atrophy in the gut occurred (Figure 1D).
Figure 1 Gross observation of intestinal pathology was shown as A, B, C and D.
A: nearly normal morphology at TBI 3 h; B: intestinal dilation with large amount of yellowish effusion and thin edema-tous wall at 72 h following TBI; C: gastrointestinal distention at 72 h following TBI, stomach indicated by solid arrow and intestine by blank arrow; D: gastrointestinal atrophy on day 7 following TBI.
Levels of VIP, CCK and CGRP in plasma
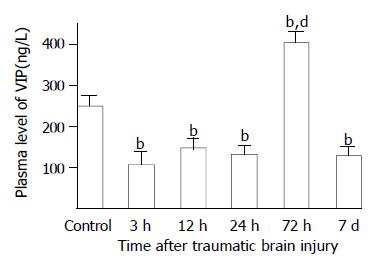
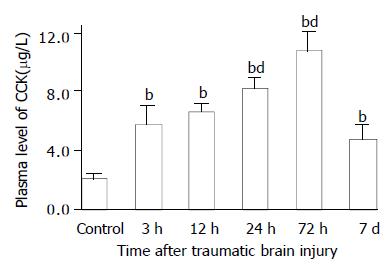
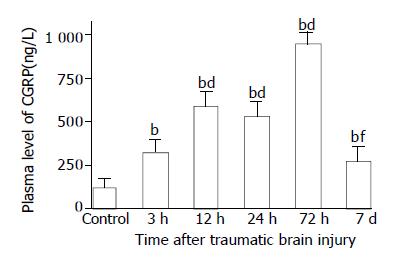
As compared with that of control group (247.8 ± 29.5 ng/L), plasma VIP levels were significantly decreased at 3, 12 and 24 h postinjury (106.7 ± 34.1 ng/L, 148.7 ± 22.8 ng/L, 132.8 ± 21.6 ng/L, respectively), but significantly increased at 72 h (405.0 ± 29.8 ng/L) and declined markedly on d 7 (130.7 ± 19.3 ng/L) (Figure 2). However, Plasma levels CCK and CGRP were significantly increased through 3 h and 7 d following TBI (126-691% increases), with the peak at 72 h (Figures 3 and 4).
Figure 2 Alteration of VIP in plasma after TBI.
Compared with control, plasma level of VIP was significantly decreased at 3, 12 and 24 h postinjury, but was significantly increased at 72 h, then declined to a low level on d 7 with the value similar to that of groups of 3 h, 12 h and 24 h. bP < 0.01 vs control; dP < 0.01 vs 3 h, 12 h, 24 h and 7 d.
Figure 3 Alteration of CCK in plasma after TBI.
Compared with control, plasma level of CCK was significantly increased postinjury, and peaked at 72 h, then declined to some degree on d 7, but was still significantly higher than that of control. bP < 0.01 vs control; dP < 0.01 vs 3 h. Mean ± SD of 6 animals, control: 2.1 ± 0.3 µg/L, 3 h: 5.8 ± 1.2 µg/L, 12 h: 6.7 ± 0.5 µg/L, 24 h: 8.3 ± 0.7 µg/L, 72 h: 10.8 ± 1.2 µg/L, 7 d: 4.8 ± 0.9 µg/L.
Figure 4 Alteration of CGRP in plasma after TBI.
Compared with control, plasma level of CGRP was significantly increased postinjury, and peaked at 72 h, then declined obviously on d 7 with the value still significantly higher than that of control. bP < 0.01 vs control; dP < 0.01 vs 3 h; fP < 0.01 vs 12 h, 24 h and 72 h. Mean ± SD of 6 animals, control: 120.8 ± 47.7 ng/L, 3 h: 323.8 ± 75. 9 ng/L, 12 h: 596.7 ± 82.7 ng/L, 24 h: 536.0 ± 77.2 ng/L, 72 h: 955. 0 ± 63.0 ng/L, 7 d: 273.3 ± 83.8 ng/L.
Levels of VIP, CCK and CGRP in jejunum
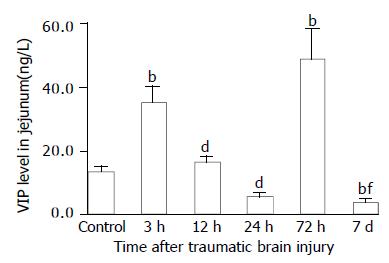
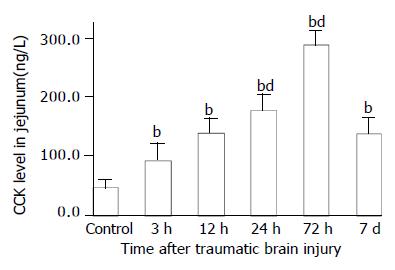
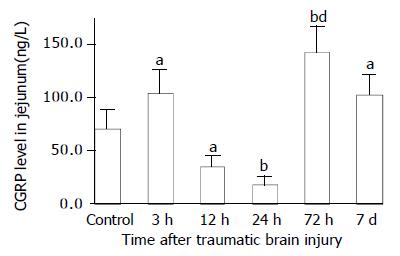
As compared with that of control group (VIP, 13.6 ± 1.4 ng/g; CGRP, 70.6 ± 17.7 ng/g), VIP and CGRP levels in jejunum were increased significantly at 3 h after TBI (VIP, 35.4 ± 5.0 ng/g; CGRP, 103.8 ± 22.1 ng/g, P < 0.01), then declined gradually at 12 h and 24 h (VIP, 16.5 ± 1.8 ng/g, 5.5 ± 1.4 ng/g; CGRP, 34.9 ± 9.7 ng/g, 18.5 ± 7.7 ng/g), but were significantly increased again at 72 h postinjury (VIP, 48.7 ± 9.5 ng/g; CGRP, 142.1 ± 24.3 ng/g, P < 0.01). The VIP level declined to a significantly low level on d 7 (3.8 ± 1.1 ng/g) (Figure 5), otherwise, CGRP declined to the level close to that of TBI 3 h group (102.5 ± 18.1 ng/g) (Figure 7). The CCK levels in jejunum were found to change in a similar trend as that in plasma with the concentrations of CCK significantly increased (99%-517% increases) from 3 h to 7 d following TBI and peaked at 72 h (Figure 6).
Figure 5 Alteration of VIP levels in all layers of jejunum after TBI.
Compared with control, the level of VIP was significantly increased at 3 h following TBI, then declined markedly at 12 h and 24 h, and was significantly increased again at 72 h, but was at its lowest level on day 7 postinjury. bP < 0.01 vs control; dP < 0.01 vs 3 h; fP < 0.01 vs 3 h and 12 h.
Figure 6 Alteration of CCK levels in all layers of jejunum after TBI.
The level of CCK was significantly increased after TBI as compared with that of control, and peaked at 72 h postinjury, then declined to some degree but was still signifi-cantly higher than that of control on d 7. bP < 0.01 vs control; dP < 0.01 vs 3 h. Mean ± SD of 6 animals, control: 46.5 ± 13.9 ng/g, 3 h: 92.6 ± 28.7 ng/g, 12 h: 139.8 ± 24.7 ng/g. 24 h: 178.5 ± 25.8 ng/g, 72 h: 286.8 ± 25.9 ng/g, 7 d: 137.8 ± 27.3 ng/g.
Figure 7 Alteration of CGRP levels in all layers of jejunum after TBI.
Compared with control group, the level of CGRP was significantly increased at 3 h, then declined significantly at 12 h and 24 h, and significantly increased again at 72 h postinjury, then declined on d 7, but was still significantly higher than that of control. aP < 0.05 vs control; bP < 0.01 vs control; dP < 0.01 vs 3 h, 12 h, 24 h and 7 d.
DISCUSSION
TBI is a critically ill condition resulting in metabolic and immune alterations, inflammatory response and disturbance of gastrointestinal peptides via hypothalamo-pituitary-adrenal axis and brain-gut axis[10,26]. Therefore, TBI can lead to disturbance of peptidergic mediators as a result of systemic stress with the same mechanism as occurred in trauma, sepsis, surgery and burns[11-17]. Brain-gut peptides possess many biological functions such as dilating vessels, improving focal blood flow perfusion, regulating immune action and relaxing smooth muscle of trachea[13,27,28]. The present study shows that brain-gut peptides VIP, CCK and CGRP change significantly following TBI in both plasma and jejunum, behave in different manner and partly adapt to the systemic pathophysiological needs. Our data suggest that levels of CCK in plasma and jejunum tissue change in parallel and increase gradually following TBI, with a maximum at 72 h postinjury. By contrast, VIP and CGRP levels in plasma and in jejunum tissue show non-parallel fluctuations. Nevertheless, concentrations of all neurohormonal mediators peak at 72 h postinjury, which coincides with the maximal morphological alterations occurring in the gut following TBI[29]. High level of VIP, CCK and CGRP in plasma implies a large amount of release of these peptides into circulation from nerve ending due to systemic stress. Decreased VIP and CGRP level in plasma and/or jejunum may be related to the low synthesis, depletion of secretion, more binding to the relevant receptors, reduced tissue perfusion of blood flow and cell edema.
On the other hand, obvious alterations of VIP, CCK and CGRP may play an important role in gastrointestinal dysmotility induced by TBI, mainly because brain-gut peptides involved in the endocrine and enteric nervous systems as well as in the central nervous system are the important factors in the regulation of gastrointestinal motility through pathways of endocrine, paracrine or neurocrine. CCK exerts action mainly via endocrine, otherwise, VIP and CGRP mainly via neurocrine[9]. Mechanic and chemical stimuli which induce peptide release from the epithelial endocrine cells are the earliest step in the initiation of peristaltic activities. The dorsal vagal complex (DVC), located in the medulla of brainstem, constitutes the basic neural circuit of vago-vagal reflex control of gastrointestinal motility[9]. Several gut peptides act on the DVC to modify the vagal cholinergic reflexes directly (VIP and CGRP) or indirectly via the afferent fibers in the peripheral (CCK). The DVC is also a primary site of action of many neuropeptides in mediating gastrointestinal motor activities (TRH and NPY). More and more evidences have shown that there is a complicated bidirectional inter-relationship between nervous system and gastrointestinal tract[30]. Gastrointestinal signals produced during chronic stress and colitis can be transmitted into central nervous system (CNS) and influence the function of the latter. In turn, CNS can modulate the electrical activity of gastrointestinal tract, as occurred in TBI.
VIP is a 28 amino acid neurotransmitter peptide that is widely distributed, particularly in the central and peripheral nervous systems, with a broad spectrum of biological actions mediated by stimulation of parasympathetic nerves coinciding with tissue responses. VIP is one of main inhibitory nervous transmitters located in the gut, which induces relaxation of intestinal smooth muscle and sphincters, dilation of peripheral blood vessels and inhibition of gastric acid secretion. Additionally, in vivo studies showed VIP could protect lung, retina, heart, kidney and stomach from the harmful effects of inflammation, ischemia-reperfusion injury and glutamate-induced cell death[27,31]. It also increases survival rate of animals exposed to severe septic and hemorrhagic shock[32]. VIP is a nonadrenergic noncholinergic nervous transmitter, and exerts inhibitory action on gastrointestinal motility through its binding to the relevant receptors located in gastrointestinal tract and subsequent activation of cyclic AMP-dependent protein kinase. VIP neurons occupy 40% of submucosal neurons of rat small intestine[33]. In the intestine, VIP markedly stimulates intestinal secretion of electrolytes and hence of water. The results of this study showed that VIP level in both plasma and jejunum was increased significantly at 72 h following TBI. Increased concentrations of VIP in plasma and jejunum might be responsible for the intestinal dilation and large amounts of intra-intestinal effusion as shown in Figure 1 B and C. From 12 h to 24 h following TBI, VIP concentrations in both plasma and jejunum were decreased significantly due to binding to receptors and low synthesis, which was partially responsible for the acute gastrointestinal mucosal damage induced by ischemia.
CCK is involved in the endocrine cells in the upper small intestine, principally acting to stimulate gallbladder contractions[34] and pancreatic secretion[35], and to delay gastric emptying after meals[26]. Evidence has shown that CCK, stimulated by nutrients, is a physiological mediator which inhibits gastric emptying and subsequently suppresses food intake[26]. CCK mainly reduces the tone of lower esophageal sphincter, inhibits the peristalsis of proximal duodenum and promotes the peristalsis of distal duodenum and jejunum via activation of Ca2+-dependent protein kinase. Previous studies have demonstrated that CCK could increase the mean contractile amplitude of antral circular muscle, motility index of pyloric circular muscle and the resting tension of fundus[21,24,36,37]. Therefore, CCK possesses both excitatory and inhibitory action on contractile activity of different regions of stomach. Since systemic administration of CCK increases vagal afferent activity[27] and this effect is blocked by the disruption of vagal capsaicin-sensitive afferent fibers, it has been suggested that CCK acts on the vagal afferent neurons and induces gastric relaxation[28] and CCK-A receptor mediates this action[30,38]. CCK exerts an inhibitory function on the colonic motility, which is mediated by CCK-A receptors[39]. Besides the effects of CCK on the digestive tract, other biological actions of this peptide have been observed, such as appetite inhibition[40], prevention of LPS-induced decrease of blood pressure and attenuation of LPS-induced increase of proinflammatory cytokines (TNF-α, IL-1β and IL-6) in vivo[12]. The current study showed that the levels of CCK in both plasma and jejunum were increased significantly following TBI, suggesting that CCK may play an important role in protection of splanchnic functions. Conversely, increased concentrations of CCK in plasma and jejunum might be highly responsible for the gastric emptying dysfunction[20,41-44] and esophageal reflux which usually occur after head trauma[1,6,7] through the mechanism as mentioned above. Gastric distention was found in rats at 12, 24 and 72 h following TBI, as shown in Figure 1, possibly due to the inhibitory action of persistently high CCK concentrations on the stomach contraction.
CGRP is a 37 amino acid neurotransmitter peptide that is widely distributed in the central and peripheral nervous systems[11,28,45], particularly in the viscera and vascular adventitia. It can also be synthesized and released by lymphocytes in rats[46]. CGRP in blood mainly comes from the gut and vessels. CGRP may be released extensively from the nerve ending around vessels into circulation through organ stimuli such as endotoxin, inflammatory mediators, stress and intestinal ischemia-reperfusion injury in the rat [11,13,47]. Endotoxemia, inflammatory cytokines and other kinds of stress can also stimulate the synthesis of CGRP in spinal dorsal root ganglia neurons (DRG). Therefore, TBI can also induce the extensive release of CGRP into plasma and synthesis of CGRP in jejunum as demonstrated in this study. CGRP possesses extensive biological functions such as cardiotonic effect, dilating vessels and regulating immune system[11]. It has been previously shown that CGRP is one of the important mediators involved in the pathogenesis of infection, hemorrhage and trauma, and also is one of the mediators for the interactions of neural-immune systems. Recently, the involvement of CGRP in abdominal surgery-induced inhibition of gastric and colonic motility has been reported[15]. The postoperative gastric ileus is mediated by CGRP released from spinal afferent neurons in the celiac and superior mesenteric ganglia as part of an extra-spinal gastrointestinal inhibitory reflex pathways[15,16]. This study demonstrated that the CGRP concentration in plasma was increased persistently after TBI. Increased level of CGRP in plasma following TBI may contribute to the decreased tone of lower esophageal sphincter, delayed gastric emptying and paralytic ileus of small intestine. Decreased concentrations of CGRP in jejunum may be responsible for the ischemic damage of gut mucosa. The mechanism of CGRP inhibiting gastrointestinal motility includes: (1) CGRP is released into local gut tissue or circulation, and then binds to the relevant receptors located on the surface of gastrointestinal smooth muscle to exert inhibitory action; (2) CGRP, acting as a part of inhibitory spinal reflex, is released into local gut induced by all kinds of stress factors to activate spinal afferent nerve fibers, leading to decreased gastrointestinal motility[15]; (3) CGRP can promote the release of substance P which partly mediate postoperative intestinal ileus[17]; (4) CGRP interacts with the receptors on surface of D cells to induce the release of somatostatin, inhibiting gastrointestinal motility; (5) CGRP may slow the transmit of cholinergic nerve fibers of enteric nervous system to inhibit gastrointestinal peristalsis.
It is concluded that the concentrations of VIP, CCK and CGRP in both plasma and jejunum change significantly following TBI. All these data imply that alterations of these peptide concentrations in plasma and gut may be involved in the pathogenesis of gastrointestinal dysfunction after traumatic brain injury. Increased CCK level might be responsible for the delayed gastric emptying, otherwise, VIP and CGRP might be responsible for the paralytic dilation of small intestine and large amounts of intra-intestinal effusion.