Published online Mar 15, 2004. doi: 10.3748/wjg.v10.i6.864
Revised: January 23, 2004
Accepted: February 28, 2004
Published online: March 15, 2004
AIM: To investigated the effects of intravenous administration of the antioxidant glutathione (GSH) on reperfusion injury following liver transplantation.
METHODS: Livers of male Lewis rats were transplanted after 24 h of hypothermic preservation in University of Wisconsin solution in a syngeneic setting. During a 2-h reperfusion period either saline (controls, n = 8) or GSH (50 or 100 μmol/(h·kg), n = 5 each) was continuously administered via the jugular vein.
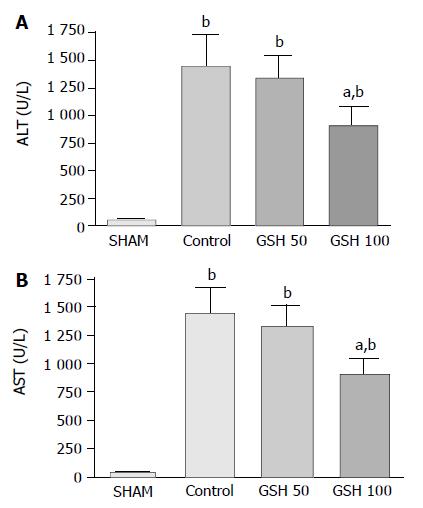
RESULTS: Two hours after starting reperfusion plasma ALT increased to 1 457 ± 281 U/L (mean ± SE) in controls but to only 908 ± 187 U/L (P < 0.05) in animals treated with 100 μmol GSH/(h·kg). No protection was conveyed by 50 μmol GSH/(h·kg). Cytoprotection was confirmed by morphological findings on electron microscopy: GSH treatment prevented detachment of sinusoidal endothelial cells (SEC) as well as loss of microvilli and mitochondrial swelling of hepatocytes. Accordingly, postischemic bile flow increased 2-fold. Intravital fluorescence microscopy revealed a nearly complete restoration of sinusoidal blood flow and a significant reduction of leukocyte adherence to sinusoids and postsinusoidal venules. Following infusion of 50 μmol and 100 μmol GSH/(h·kg), plasma GSH increased to 65 ± 7 mol/L and 97 ± 18 mol/L, but to only 20 ± 3 mol/L in untreated recipients. Furthermore, plasma glutathione disulfide (GSSG) increased to 7.5 ± 1.0 mol/L in animals treated with 100 μmol/(h·kg) GSH but did not raise levels of untreated controls (1.8 ± 0.5 mol/L) following infusion of 50 μmol GSH/(h·kg) (2.2 ± 0.2 mol/L).
CONCLUSION: Plasma GSH levels above a critical level may act as a “sink” for ROS produced in the hepatic vasculature during reperfusion of liver grafts. Therefore, GSH can be considered a candidate antioxidant for the prevention of reperfusion injury after liver transplantation, in particular since it has a low toxicity in humans.
- Citation: Schauer RJ, Kalmuk S, Gerbes AL, Leiderer R, Meissner H, Schildberg FW, Messmer K, Bilzer M. Intravenous administration of glutathione protects parenchymal and non-parenchymal liver cells against reperfusion injury following rat liver transplantation. World J Gastroenterol 2004; 10(6): 864-870
- URL: https://www.wjgnet.com/1007-9327/full/v10/i6/864.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i6.864
A decade after the introduction of the University of Wisconsin (UW) solution for cold preservation of solid organs, hepatic preservation and reperfusion injury is still a serious problem which contributes to primary nonfunction and dysfunction of the liver allograft[1-3]. Years of research in liver preservation have established the superiority of the UW formulation over other solutions. Although hepatocytes and sinusoidal endothelial cells are injured during cold preservation in UW solution they remain alive even after periods of ischemia well beyond the limit of organ viability[2-4]. Hepatocyte and SEC death occurs rapidly on oxygenated reperfusion[2,4] and the extent of SEC death has been shown to be a critical factor influencing graft survival in various animal models[5-7]. However, therapeutic strategies which reduce reperfusion injury to hepatocytes and SEC during liver transplantation remain to be established.
There is substantial evidence that activation of Kupffer cells (KC), the generation of reactive oxygen species (ROS) and disturbance of the hepatic microcirculation contribute to reperfusion injury[8-11]. During the reperfusion, activated KC produce mediators of inflammation, including tumor necrosis factor α, interleukins and chemokines, and release ROS into the sinusoidal space[3,8]. The resulting vascular oxidant stress has been discovered as potential mechanism of vasoconstriction and leukocyte adherence in the liver[12-16]. This may lead to disturbance of the hepatic microcirculation, ultimately resulting in hypoxic cell injury. Furthermore, KC-derived ROS could activate redox-sensitive transcription factors such as nuclear factor (NF)- κB and activator protein-1 (AP-1) in endothelial cells and hepatocytes, thereby activating proinflammatory genes and adding to the hepatic damage[17,18].
Thus, ROS can be considered as signal molecules which trigger several pivotal mechanisms of reperfusion injury. A large number of investigations using antioxidant interventions support this hypothesis[10]. However, the clinical relevance of many antioxidants is limited by side-effects or high cost which may explain the lack of established antioxidative interventions to prevent hepatic reperfusion injury[3]. Therefore, recent studies investigated the therapeutic potential of the endogenous antioxidant glutathione (GSH), in particular since it has a low toxicity in humans[19]. Furthermore, GSH is able to react spontaneously with nearly all oxidants formed during inflammation, which results in the formation of oxidized glutathione (GSSG)[20-22]. Previous studies demonstrated that GSH released through the GSH transporter of hepatocytes may act as an endogenous defense system against KC – and leukocyte – derived ROS, thus protecting the hepatic vasculature from damage by inflammatory cells[8]. Therefore, interventions increasing plasma GSH levels should confer protection against reperfusion injury. Accordingly, treatment of cold preserved livers with GSH upon reperfusion prevented damage to hepatocytes in the model of isolated rat liver perfusion[23]. These in vitro findings suggest that GSH, as a candidate drug, could prevent reperfusion injury of the liver allograft. Consequently, future studies need to be directed toward characterization of the protective potential of GSH treatment in vivo which may have important implications for an application in the clinical setting.
Therefore, the purpose of this study was to investigate whether intravenous administration of GSH protects the rat liver against reperfusion injury during liver transplantation. In particular, the aims of the current study are: (1) to investigate effects of intravenously applied GSH on hepatocyte and SEC damage; (2) to examine the effect of GSH treatment on disturbances of the hepatic microcirculation, and (3) to determine the functional significance of changes of the extracellular antioxidant capacity.
Syngeneic, male Lewis rats (donors: 207 ± 12 g; recipients: 276 ± 18 g body mass) were purchased from Charles River Wiga, Sulzfeld, Germany and housed in a temperature- and humidity-controlled room under a constant 12-h light/dark cycle. Animals had free access to water and rat chow (standard diet, Altromin, Germany). All experiments were performed with rats fasted 12 h prior to donor and recipient operations. All studies were performed with the permission of the government authorities and in accordance with the German Legislation on Laboratory Animal Experiments.
Donor and recipient operations were performed under spontaneous ether inhalation. The left carotid artery was cannulated with a polyethylene catheter for continous monitoring of the mean arterial blood pressure and heart rate during the operation. Another catheter was inserted into the jugular vein for substitution of plasma volume and injection of fluorescent compounds as well as for continuous infusion of glutathione during reperfusion. Body temperature was kept between 36.5 °C and 37.5 °C by means of a heating pad. Donor livers were preserved by retrograde aortal flush with 10 mL UW-solution and stored at 4 °C for 24 h. Prior to the implantation procedure, the livers were rinsed with warm Ringer’s lactate solution (10 mL) via the portal vein at a hydrostatic pressure of 10 cm H2O. Orthotopic liver transplantation was performed using a modified cuff technique[24]. Differing from the original technique, grafted livers were rearterialized and simultaneously reperfused through the portal vein and hepatic artery[25]. Portal clamping time was less than 20 min in all experiments.
The common bile duct of the graft was cannulated with a PE-tube and bile was collected in Eppendorf cups. Plasma samples (500 µL) were obtained in the recipient before hepatectomy and 60 and 120 min after reperfusion of the transplanted liver. The volume of the blood drawn was replaced by saline. Five minutes after starting reperfusion, all rats received 0.5 mL of albumin (5%) and 0.5 mL sodium bicarbonate in order to maintain blood pressure and physiological pH. To avoid major fluid loss and drying of the liver, possibly affecting microhemodynamics the abdominal cavity was covered with Saran wrap throughout the operation. After 120 min of reperfusion, experiments were terminated and the liver weight was determined.
Two intervention groups (n = 5 each) were compared with the control group (n = 8). In control animals, saline was infused intravenously (2 mL/h) during reperfusion of the grafted liver, starting 20 min before declamping of the portal vein and hepatic artery. In contrast, intervention groups received GSH via the jugular vein, starting 20 min prior to reperfusion until the end of the two hour reperfusion period (total infusion time 140 min). GSH (Tationil 600®, Roche Pharmaceuticals/Italy) was administered at 50 or 100 µmol/(h·kg) (n = 5 each) by continuous infusion (2 mL/h) of stock solutions (0.24 mol/L and 0.48 mol/L), using a micro-infusion pump (SP100i, WPI, Aston, UK). Two sham groups underwent laparotomy and intravital microscopy without hepatic ischemia in the absence (n = 6) or presence of intravenous GSH infusion (100 µmol GSH/(h·kg), n = 5) for 140 min.
In vivo fluorescence microscopic studies were performed 30 min after revascularization under stable hemodynamic conditions, using a modified Leitz-Orthoplan microscope combined with epi-illumination technique[26,27]. For assessment of microvascular liver perfusion and leukocyte-endothelial interaction the left liver lobe was exteriorized on a stage. For visualization of microcirculatory disturbances, sodium fluorescein (1 µmol/kg; Merck AG, Darmstadt, Germany) and rhodamin 6G (0.1 µmol/kg; Merck AG, Darmstadt, Germany) were injected intravenously for fluorescent staining of hepatocytes (negative contrast for plasma) and leukocytes, respectively. Quantification of microhemodynamic parameters was performed offline by frame-to-frame analysis of the videotaped images in a blinded fashion. Lobular perfusion and leukocyte adherence were analyzed by scanning a region of 10 randomly selected acinar areas and postsinusoidal venules, respectively. Non – perfused sinusoids were estimated by counting the number of continuously perfused and non - perfused sinusoids and were expressed as the percentage of all sinusoids visible in a pre-defined area. Permanent adherence (sticking) of leukocytes to the sinusoidal endothelium was quantified by counting the number of permanently attached leukocytes (at least for 20 s) within the 3 different segments of sinusoids. Leukocyte sticking in postsinusoidal venules was determined by the quantity of leukocytes attached for at least 20 s to the surface of postsinusoidal venules. Furthermore, temporary adherence of leukocytes (rolling) was assessed as the number of transiently attached leukocytes along the endothelial surface of postsinusoidal venules during an observation period of 20 s.
KC function was assessed by determination of their particle phagocytosis as described earlier[28,29]. At the end of IVM analysis, plain fluorescent latex beads in isotonic saline solution were injected as bolus (3×108/kg) through the carotid catheter (diameter 1.1 µm, Polysciences Inc., Warrington, PA, USA). Phagocytic activity was then analyzed successively during the first 5 min after injection in 10 randomly selected liver lobules in each experiment. Adherence of latex particles was quantified by counting the number of beads moving in sinusoids as a percentage of all beads visible in the field during an observation period of 10 s. Beads in presinusoidal and postsinusoidal venules were not included in analysis.
Blood samples of 500 µL were collected for determination of total glutathione (sum of GSH and GSSG) and GSSG. For GSSG analysis, an aliqot (200 µL) of blood was mixed immediately with 200 µL of 10 mmol/L N- ethylmaleimide (NEM) in 100 mmol/L phosphate buffer (pH 6.5) containing 17.5 mmol/L EDTA[30]. The remaining blood was centrifuged at full speed for 1 min. An aliquot (100 µL) of plasma was pipetted into 100 µL sulfosalicylic acid (5%) for determination of total glutathione. To separate GSSG from NEM and NEM-GSH adducts, an aliquot of NEM treated plasma was passed through a Sep-PakC18 cartridge (Waters, Framingham, MA, USA) followed by 1 mL of 100 mmol/L phosphate buffer (pH 7.5). GSSG in the eluates and total glutathione in acidified plasma samples was determined by an enzymatic test as described previously[31]. GSH plasma concentrations were calculated as the difference between total glutathione and GSSG.
Serum aminotransferases were used as established markers of hepatic injury. Aspartarte aminotransferase (AST) and alanine aminotransferase (ALT) were measured 2 h after reperfusion using a serum multiple analyzer (Hitachi 917, Roche Germany).
At the end of the experiments, perfusion-fixation of hepatic tissue was performed using a 50 g/L glutaraldehyde/40 g/L paraformaldehyde mixture in phosphate buffer (pH 7.4) via the hepatic artery at a constant pressure of approximately 80 mmHg. Specimens were cut from both the right and left liver lobes and blocks were diced into 1 mm cubes. Samples were stored in fixative for 2 to 3 d prior to further processing. Specimens were postfixed with osmium tetroxide, dehydrated in graded alcohol and embedded in Epon 812. Ultrathin sections were cut and contrasted with uranyl acetate and lead citrate for electron microscopy[11].
All data are expressed as mean ± SE. Statistical differences between groups were calculated using paired or unpaired Student‘s t test for randomly distributed data and the Mann-Whitney U test for nonparametric data following analysis of variance (ANOVA). Differences were considered significant at P < 0.05.
Injury of parenchymal and non-parenchymal liver cells after liver transplantation was assessed by the release of ALT and AST as well as by electron microscopic analysis at 120 min of reperfusion. Parenchymal cell damage in untreated animals was indicated by a 25- and 40-fold increase of ALT and AST serum levels, respectively, when compared with sham- operated animals (Figure 1). Continuous intravenous administration of 100 µmol GSH/(h·kg) during reperfusion significantly (P < 0.05) reduced ALT and AST levels by almost 40% whereas infusion of 50 µmol GSH/(h·kg) had no effect (Figure 1).
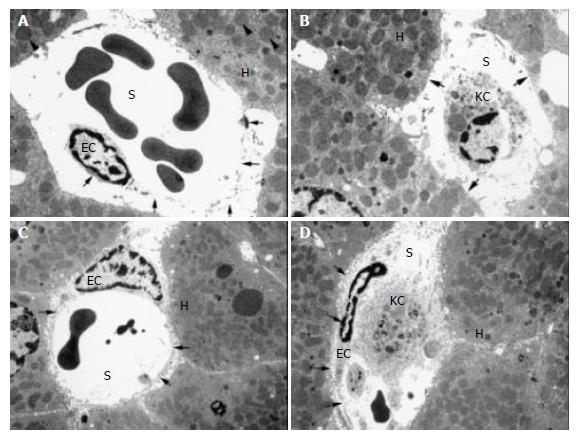
Transmission electron microphotographs of the untreated liver grafts after 120 min of reperfusion showed alterations of hepatocytes, including cell edema, a substantial loss of microvilli and generalized swelling of mitochondria (Figure 2A,B). Furthermore, non- parenchymal injury was evident by various degrees of SEC–detachment from the perisinusoidal matrix plate or even complete loss of the sinusoidal endothelial lining (Figure 2 A,B). Consequently, the space of Dissé was enlarged in control livers. In contrast, treatment of animals with 100 µmol/(h·kg) GSH was effective in protecting hepatocytes with only minimal loss of microvilli of normally shaped parenchymal cells and only sporadic mitochondrial swelling. Moreover, GSH prevented SEC detachment, thus resulting in normal spaces of Dissé (Figure 2 C,D).
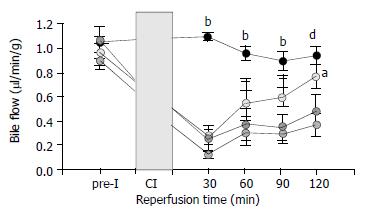
Function of liver allografts was estimated by recovery of bile flow. Bile flow of sham-operated animals remained constant during the observation period of 150 min and was comparable to bile flow rates of donor livers before ischemia (Figure 3). After transplantation of control livers, bile flow returned to only 45% of baseline values. Intravenous infusion of 50 µmol GSH/(h·kg) had no effect on the postischemic bile flow whereas 100 µmol GSH/(h·kg) significantly (P < 0.05) increased bile flow during the complete reperfusion period (Figure 3).
Latex particle phagocytosis by KC reflects their function under various conditions. In agreement with earlier observations, rapid clearance of intra-arterially administered fluorescent latex particles was observed in sham-operated animals. Five minutes after injection only 8.1 ± 2.5% particles were freely movable in the sinusoids. This rate was not influenced by liver transplantation as indicated by 11.2 ± 1.0% of all visible beads, freely movable in the sinusoids of untreated animals. Neither GSH treatment with 50 µmol/(h·kg) nor with 100 µmol/(h·kg) affected phagocytosis of latex particles, resulting in 12.0 ± 1.1% and 10.5 ± 2.0% of beads moving in sinusoids at 5 min upon bolus injection, respectively. Since KC represents approximately 90% of the phagocytic capacity in the liver, these results suggest unaltered KC function after reperfusion of untreated and GSH- treated liver grafts.
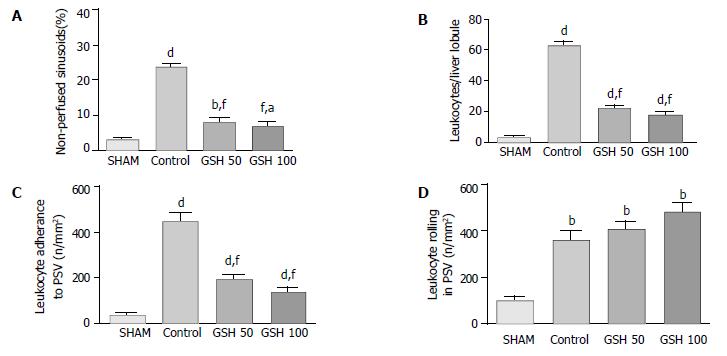
Compared with sham-operated animals, substantial disturbances of the microcirculation were observed in untreated liver allografts (Figure 4). Thirty minutes after starting reperfusion almost 25% of the sinusoids were not perfused. Intravenous infusion of 50 as well as 100 µmol GSH/(h·kg) prevented this no – reflow phenomenon, thereby improving hepatic perfusion (Figure 4 A).
Furthermore, intravital microscopy revealed considerable leukocyte sticking within sinusoids and postsinusoidal venules in untreated liver allografts (Figure 4 B,C). A significant reduction (P < 0.001) of stagnant leukocytes in sinusoids was found in both treatment groups (Figure 4 B). Comparable to acinar leukocyte sticking, permanent adherence of leukocytes to postsinusoidal venules was reduced by 57% and 69% (P < 0.001) when animals received 50 or 100 µmol GSH/(h·kg), respectively (Figure 4 C). Temporary adherence of leukocytes (rolling) in postsinusoidal venules is supposed to be the initiating event in the sequence to permanent adherence to the vascular endothelium. However rolling of leukocytes was slightly increased by GSH treatment (Figure 4E).
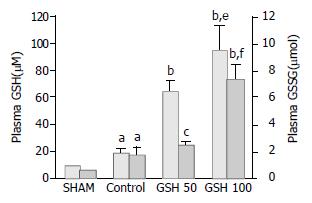
Sixty minutes after starting reperfusion plasma GSH levels of untreated animals increased 2-fold when compared to sham-operated animals (Figure 5). This was accompanied by a 3-fold increase of plasma GSSG (Figure 5). Infusion of 50 µmol GSH/(h·kg) resulted in a 6-fold increase of plasma GSH whereas GSSG did not exceed levels of untreated controls. In contrast, 100 µmol GSH/(h·kg) elevated plasma GSH as well as GSSG by up to 9-fold above values obtained in the sham-group (Figure 5). Moreover, plasma GSSG increased to significant higher levels when compared with untreated or GSH–treated (50 µmol/(h·kg)) animals. In order to estimate the role of spontaneous oxidation of intravenously applied GSH a second group of sham-operated animals was treated with 100 µmoL GSH/(h·kg). This resulted in marked increase of plasma GSH to 93 ± 10 mol/L which was similar to that observed in liver allografts (97 ± 18 mol/L). However, plasma GSSG increased to only 3.0 ± 0.9 in contrast to 7.5 ± 0.9 mol/L in GSH-treated liver allografts. These findings clearly indicate enhanced oxidation of intravenously applied GSH at the cytoprotective dose of 100 µmol/(h·kg).
Recently, we have found that treatment of cold preserved livers with GSH upon reperfusion prevented reperfusion injury in the model of isolated rat liver perfusion[23]. Due to several limitations of this experimental model, such as the absence of plasma antioxidants and the lack of leukocytes[32,33], the potential protective effect of GSH treatment during liver transplantation remained to be defined. We also found that intravenous GSH treatment during liver transplantation prevents reperfusion injury of the liver allograft.
We obtained the following main results: (1) increase of the vascular antioxidant capacity by intravenous infusion of GSH causes a significant reduction of injury to hepatocytes and SEC as well as of (2) microcirculatory disturbances in liver allografts, and (3) Administration of GSH does not affect KC function but enhances detoxification of KC-derived ROS as indicated by a marked increase of plasma GSSG.
Intravenous administration of GSH at a dose of 100 µmol/h/kg during the reperfusion phase of liver transplantation significantly reduced ALT and AST release by approximately 40% indicating the protection of the liver allografts. This contention is supported by distinct signs of parenchymal and non-parenchymal cell injury under electron microscopy already after 2 h of re-establishing blood flow. Untreated livers showed a complete detachment of SEC from the perisinusoidal matrix which in part determines graft viability[4-7]. In contrast, detachment of SEC was dramatically attenuated in GSH-treated livers, indicating preservation of the sinusoidal architecture. Furthermore, GSH treatment markedly reduced mitochondrial swelling and the loss of microvilli in hepatocytes. These cytoprotective effects were accompanied by a marked improvement of postischemic liver function as assessed by a 2.5-fold increase of bile flow. Because postischemic GSH treatment can only protect cells which are not already seriously damaged before the onset of reperfusion, our results clearly demonstrate prevention of reperfusion injury to parenchymal and non-parenchymal liver cells by GSH.
Prevention of reperfusion injury by GSH depended on the GSH dose: 100 µmol/(h·kg) but not 50 mmol/(h·kg) showed protection. Based on steady state GSH plasma concentrations following GSH infusion, a concentration of approximately 100 mol/L appears to be pivotal for cytoprotection. In view of these findings, GSH seems as ideal and presumably safe candidate drug for prevention of reperfusion injury of the liver allograft since plasma GSH levels up to 500 mol/L showed no toxicity in humans[19].
Effect on Kupffer cell function There is extensive experimental evidence for KC activation as a central pathomechanism of reperfusion injury of the liver after warm or cold ischemia[8,9]. To assess KC function we used the tool of intravital microscopic analysis of latex particle phagocytosis[28,29]. In agreement with earlier studies[29] particle phagocytosis was not affected by cold preservation indicating functionally intact Kupffer cells in liver allografts. Furthermore, clearance of latex particles was not affected by postischemic administration of GSH. These results argue against a suppression of KC function as a potential protective mechanism of GSH action. Thus, potential hazards of other protective approaches interfering with vital host-defense function of KC (e.g. calcium channel blocker, KC-depleting agents;[8,34,35]) would be avoided by GSH treatment. Detoxification of ROS in the hepatic vasculature Intravenous administration of GSH results in the degradation of GSH to its amino acids which are the again available for GSH synthesis in various organs including the liver[36]. However several studies demonstrated no relevant increase of the hepatic GSH content 2 h after starting high-dose GSH infusion[22,23]. These findings suggest that GSH effects are related rather to the increase in the extracellular antioxidant capacity than to influences on intracellular GSH. In line with this interpretation we obtained evidence for an extracellular mechanism of GSH-mediated cytoprotection.
The increase in plasma GSSG observed during reperfusion of untreated and GSH-treated livers was associated with a marked increase in plasma GSH. This parallel increase in GSH and GSSG suggests that GSSG resulted from extracellular oxidation rather than from intrahepatic generation of GSSG[37,38]. Accordingly, previous studies with the blood – free perfused rat liver found only a very small release of GSSG into the perfusate during reperfusion[39]. Furthermore, there was no evidence for a relevant increase of intracellular GSSG formation[8,39]. Thus, plasma GSSG formation observed during reperfusion of liver allografts is likely to take place extracellularly. This could be due to spontaneous as well as reactive oxygen species-related oxidation of GSH. The results of the present study argue against a predominant role for spontaneous GSH oxidation. First, intravenous infusion of 50 µmol GSH/(h·kg) during reperfusion of liver allografts resulted in a several fold increase of plasma GSH whereas plasma GSSG did not exceed the levels of untreated controls. Second, a comparable increase of plasma GSH was observed when sham-operated or transplanted animals were treated with 100 µmol GSH/(h·kg). In contrast, a 2.5-fold higher increase of plasma GSSG was determined in transplanted rats. This difference clearly indicates that spontaneous oxidation of intravenously applied GSH contributes only in part to the increase in plasma GSSG. Based on these findings oxidation of GSH by ROS appears as a major determinant of plasma GSSG formation following liver transplantation. Consequently, the marked increase of plasma GSSG during infusion of the protective GSH dose of 100 µmol/(h·kg) most likely reflects an enhanced detoxification of ROS in the hepatic vasculature.
Activated KC have been identified as potential source of extracellular ROS formation and GSH oxidation during reperfusion after warm hepatic ischemia[8,40]. Accordingly, there was no evidence for plasma GSSG formation during reperfusion of KC-depleted liver allografts[41]. These findings suggest that intravenously applied GSH protects liver allografts against the vascular oxidant stress produced by KC during reperfusion. This interpretation is further substantiated by the observation that externally added GSH and catalase protected leukocyte- free perfused rat livers against oxidant cell injury following selective KC activation[42,43]. In this experimental model cytoprotection was achieved by vascular GSH concentrations of 100 mol/L, but not by 50 mol/L. The same vascular concentrations of GSH were achieved in the present study. Again, protection of liver allografts was only evident at plasma GSH concentrations of approximately 100 mol/L and was associated with a considerable extra-formation of plasma GSSG. These findings suggest that plasma GSH above a critical level may act as a “sink“for ROS produced by KC during reperfusion. This could be due to non-enzymatic reactions between GSH and various ROS which strongly depend on the GSH concentration[29,44,45].
Prevention of microcirculatory perfusion failure and SEC injury GSH of both doses remarkably improved the hepatic microcirculation. In particular, the number of perfused sinusoids increased to that observed in non-ischemic livers indicating prevention of the no-reflow phenomenon. This could be related to the counteraction of ROS-mediated mechanisms of vasoconstriction and leukocyte adherence to sinusoids and postsinusoidal venules[12-16]. In accordance with the proposed stepwise process of leukocyte-endothelial interaction from leukocyte rolling as an initial event to permanent attachment of leukocytes as the second step[46], our results indicate GSH as an inhibitor of the second step: treatment with GSH prevented sticking but slightly increased the rolling of leukocytes. Similar effects can be achieved by the administration of superoxide dismutase[12,13,16], suggesting superoxide anion (O2-•) as the contributing mediator of leukocyte adherence. Therefore, it seems likely that GSH prevents leukocyte adherence through reaction with O2-• or inhibition of O2-• - mediated mechanisms of leukocyte adherence. Protection of leukocyte adherence by 50 µmoL GSH/ (h·kg) without evidence for additional GSSG formation does not argue against this concept because the reaction of GSH with O2-• can result in the formation of glutathione sulfonate[44].
However, the antioxidative potential of GSH does not rule out beneficial effects through non-antioxidative properties. Accumulating evidence indicates that detachment of SEC from the perisinusoidal matrix plate represents a critical component of preservation injury and postischemic microcirculatory failure[5,47,48]. It has been proposed that SEC damage occurs independent of ROS formation because several antioxidants failed to prevent SEC injury in the liver allograft[6]. SEC detachment is caused by various matrix metalloproteinases (MMPs) released into the extracellular compartment during cold liver preservation. In the present study attachment of SEC to their matrix was impressively preserved by GSH and could contribute to the improvement of the hepatic microcirculation. Recent experiments demonstrated the inhibition of MMPS by GSH in vitro. These findings suggest that MMPs were possible targets of GSH in liver allografts but this requires further investigations.
In summary, the present study demonstrates a dose-dependent prevention of reperfusion injury in rat liver allografts by postischemic intravenous administration of GSH. Treatment of recipient animals with GSH prevented reperfusion injury to SEC as well as to hepatocytes, markedly improved the hepatic microcirculation and preserved postischemic liver function. GSH-mediated protection of liver allografts was associated with an increased formation of plasma GSSG providing evidence for an accelerated detoxification of ROS by intravenously applied GSH. Therefore, intravenous administration of GSH appears to be a candidate therapy for the prevention of ROS-related reperfusion injury after liver transplantation, in particular since it has a low toxicity in humans and seems cost-effective.
The excellent technical assistance of Ms I. Liss is gratefully appreciated. Presented in abstract form at the 50th annual meeting of the American Association for the Study of Liver Diseases (AASLD), 1999, Dallas.
Supported in part by a grant from the Friedrich-Baur Stiftung, the Muenchener Medizinische Wochenschrift (MMW) and the Deutsche Forschungsgemeinschaft (DFG Scha 857/1-1; DFG Ge 576/24-1)
Edited by Ma JY Proofread by Zhu LH and Xu FM
| 1. | Clavien PA, Harvey PR, Strasberg SM. Preservation and reperfusion injuries in liver allografts. An overview and synthesis of current studies. Transplantation. 1992;53:957-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 613] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 2. | Lemasters JJ, Thurman RG. Reperfusion injury after liver preservation for transplantation. Annu Rev Pharmacol Toxicol. 1997;37:327-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 236] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Bilzer M, Gerbes AL. Preservation injury of the liver: mechanisms and novel therapeutic strategies. J Hepatol. 2000;32:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 130] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Clavien PA. Sinusoidal endothelial cell injury during hepatic preservation and reperfusion. Hepatology. 1998;28:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | McKeown CM, Edwards V, Phillips MJ, Harvey PR, Petrunka CN, Strasberg SM. Sinusoidal lining cell damage: the critical injury in cold preservation of liver allografts in the rat. Transplantation. 1988;46:178-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 333] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Caldwell-Kenkel JC, Thurman RG, Lemasters JJ. Selective loss of nonparenchymal cell viability after cold ischemic storage of rat livers. Transplantation. 1988;45:834-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 134] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Gao W, Bentley RC, Madden JF, Clavien PA. Apoptosis of sinusoidal endothelial cells is a critical mechanism of preservation injury in rat liver transplantation. Hepatology. 1998;27:1652-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 243] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355-G362. [PubMed] |
| 9. | Brass CA, Roberts TG. Hepatic free radical production after cold storage: Kupffer cell-dependent and -independent mechanisms in rats. Gastroenterology. 1995;108:1167-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Jaeschke H. Reactive oxygen and ischemia/reperfusion injury of the liver. Chem Biol Interact. 1991;79:115-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 240] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Vollmar B, Glasz J, Leiderer R, Post S, Menger MD. Hepatic microcirculatory perfusion failure is a determinant of liver dysfunction in warm ischemia-reperfusion. Am J Pathol. 1994;145:1421-1431. [PubMed] |
| 12. | Koo A, Komatsu H, Tao G, Inoue M, Guth PH, Kaplowitz N. Contribution of no-reflow phenomenon to hepatic injury after ischemia-reperfusion: evidence for a role for superoxide anion. Hepatology. 1992;15:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 152] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Marzi I, Knee J, Bühren V, Menger M, Trentz O. Reduction by superoxide dismutase of leukocyte-endothelial adherence after liver transplantation. Surgery. 1992;111:90-97. [PubMed] |
| 14. | Bilzer M, Gerbes AL. Prolonged modulation of the hepatic circula-tion by Kupffer cell-derived reactive oxygen species. In Cells of the hepatic sinusoid (eds. E. Wisse, D.L. Knook, C. Balabaud), Vol. 6. Leiden, Netherlands: Kupffer Cell Foundation 1996; pp. 200-201. |
| 15. | Shibuya H, Ohkohchi N, Seya K, Satomi S. Kupffer cells generate superoxide anions and modulate reperfusion injury in rat livers after cold preservation. Hepatology. 1997;25:356-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Kondo T, Terajima H, Todoroki T, Hirano T, Ito Y, Usia T, Messmer K. Prevention of hepatic ischemia-reperfusion injury by SOD-DIVEMA conjugate. J Surg Res. 1999;85:26-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Essani NA, McGuire GM, Manning AM, Jaeschke H. Endotoxin-induced activation of the nuclear transcription factor kappa B and expression of E-selectin messenger RNA in hepatocytes, Kupffer cells, and endothelial cells in vivo. J Immunol. 1996;156:2956-2963. [PubMed] |
| 18. | Essani NA, Fisher MA, Jaeschke H. Inhibition of NF-kappa B activation by dimethyl sulfoxide correlates with suppression of TNF-alpha formation, reduced ICAM-1 gene transcription, and protection against endotoxin-induced liver injury. Shock. 1997;7:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 80] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Aebi S, Assereto R, Lauterburg BH. High-dose intravenous glutathione in man. Pharmacokinetics and effects on cyst(e)ine in plasma and urine. Eur J Clin Invest. 1991;21:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Bilzer M, Lauterburg BH. Glutathione metabolism in activated human neutrophils: stimulation of glutathione synthesis and consumption of glutathione by reactive oxygen species. Eur J Clin Invest. 1991;21:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Bilzer M, Lauterburg BH. Effects of hypochlorous acid and chloramines on vascular resistance, cell integrity, and biliary glutathione disulfide in the perfused rat liver: modulation by glutathione. J Hepatol. 1991;13:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Liu P, Fisher MA, Farhood A, Smith CW, Jaeschke H. Beneficial effects of extracellular glutathione against endotoxin-induced liver injury during ischemia and reperfusion. Circ Shock. 1994;43:64-70. [PubMed] |
| 23. | Bilzer M, Paumgartner G, Gerbes AL. Glutathione protects the rat liver against reperfusion injury after hypothermic preservation. Gastroenterology. 1999;117:200-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 531] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 25. | Post S, Menger MD, Rentsch M, Gonzalez AP, Herfarth C, Messmer K. The impact of arterialization on hepatic microcirculation and leukocyte accumulation after liver transplantation in the rat. Transplantation. 1992;54:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Vollmar B, Richter S, Menger MD. Leukocyte stasis in hepatic sinusoids. Am J Physiol. 1996;270:G798-G803. [PubMed] |
| 27. | Menger MD, Marzi I, Messmer K. In vivo fluorescence microscopy for quantitative analysis of the hepatic microcirculation in hamsters and rats. Eur Surg Res. 1991;23:158-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 78] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Bloch EH, McCuskey RS. Biodynamics of phagocytosis: an analysis of the dynamics of phagocytosis in the liver by. in vivo microscopy. In Kupffer cells and other liver sinusoidal cells (eds. E. Wisse, D.L. Knook), Amsterdam: Elsevier Science Publishers. 1977;pp. 21-32. |
| 29. | Post S, Gonzalez AP, Palma P, Rentsch M, Stiehl A, Menger MD. Assessment of hepatic phagocytic activity by in vivo microscopy after liver transplantation in the rat. Hepatology. 1992;16:803-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4526] [Cited by in RCA: 4628] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 32. | Gores GJ, Kost LJ, LaRusso NF. The isolated perfused rat liver: conceptual and practical considerations. Hepatology. 1986;6:511-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 222] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Halliwell B, Gutteridge JM. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 1990;280:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 907] [Cited by in RCA: 856] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 34. | Marzi I, Walcher F, Bühren V. Macrophage activation and leukocyte adhesion after liver transplantation. Am J Physiol. 1993;265:G172-G177. [PubMed] |
| 35. | Post S, Palma P, Rentsch M, Gonzalez AP, Menger MD. Differential impact of Carolina rinse and University of Wisconsin solutions on microcirculation, leukocyte adhesion, Kupffer cell activity and biliary excretion after liver transplantation. Hepatology. 1993;18:1490-1497. [PubMed] |
| 36. | Aebi S, Lauterburg BH. Divergent effects of intravenous GSH and cysteine on renal and hepatic GSH. Am J Physiol. 1992;263:R348-R352. [PubMed] |
| 37. | Adams JD, Lauterburg BH, Mitchell JR. Plasma glutathione and glutathione disulfide in the rat: regulation and response to oxidative stress. J Pharmacol Exp Ther. 1983;227:749-754. [PubMed] |
| 38. | Lauterburg BH, Smith CV, Hughes H, Mitchell JR. Biliary excretion of glutathione and glutathione disulfide in the rat. Regulation and response to oxidative stress. J Clin Invest. 1984;73:124-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 136] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Jaeschke H, Smith CV, Mitchell JR. Reactive oxygen species during ischemia-reflow injury in isolated perfused rat liver. J Clin Invest. 1988;81:1240-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 174] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by Kupffer cells and priming of neutrophils during reperfusion after hepatic ischemia. Free Radic Res Commun. 1991;15:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 188] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Schauer RJ, Bilzer M, Kalmuk S, Gerbes AL, Leiderer R, Schildberg FW, Messmer K. Microcirculatory failure after rat liver transplantation is related to Kupffer cell-derived oxidant stress but not involved in early graft dysfunction. Transplantation. 2001;72:1692-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Baron A, Gerbes AL, Bilzer M. Prevention of Kupffer cell- in-duced injury in rat liver by glutathione. Hepatology. 1999;30:226A. |
| 43. | Bilzer M, Jaeschke H, Vollmar AM, Paumgartner G, Gerbes AL. Prevention of Kupffer cell-induced injury in rat liver by atrial natriuretic peptide (ANP): A novel endogenous defense mecha-nism against oxidant injury. Am J Physiol. 1999;276:G1137-G1144. |
| 44. | Wefers H, Sies H. Oxidation of glutathione by the superoxide radical to the disulfide and the sulfonate yielding singlet oxygen. Eur J Biochem. 1983;137:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 175] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Winterbourn CC, Metodiewa D. The reaction of superoxide with reduced glutathione. Arch Biochem Biophys. 1994;314:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 189] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 46. | Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987;253:H699-H703. [PubMed] |
| 47. | Clavien PA, Harvey PR, Sanabria JR, Cywes R, Levy GA, Strasberg SM. Lymphocyte adherence in the reperfused rat liver: mechanisms and effects. Hepatology. 1993;17:131-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Takei Y, Marzi I, Gao WS, Gores GJ, Lemasters JJ, Thurman RG. Leukocyte adhesion and cell death following orthotopic liver transplantation in the rat. Transplantation. 1991;51:959-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 3.1] [Reference Citation Analysis (0)] |