Published online Mar 15, 2004. doi: 10.3748/wjg.v10.i6.809
Revised: December 2, 2003
Accepted: December 8, 2003
Published online: March 15, 2004
AIM: To study the relationship between hypoxia or epidermal growth factor (EGF) and the overexpression of vascular endothelial growth factor (VEGF) in hepatocellular carcinoma (HCC) and the signal transduction pathway of the transcription of VEGF in hepatoma cells.
METHODS: Cobalt chloride and recombinant human EGF were used to stimulate the hepatoma cell lines HepG2. VEGF mRNA was detected by using of semi-quantitative polymerase chain reaction (RT-PCR). Specific inhibitors of phosphatidylinositol 3-kinase (PI3K) and p42/p44 mitogen activated protein kinase (MAPK) were used to observe the effects of the two kinases on the regulation of the transcription of VEGF in hepatoma cells.
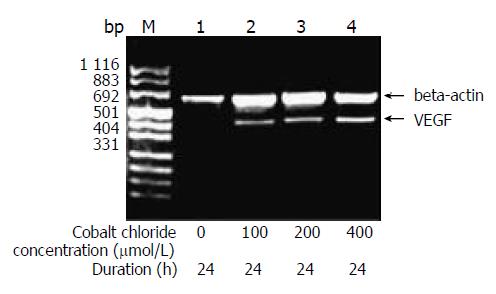
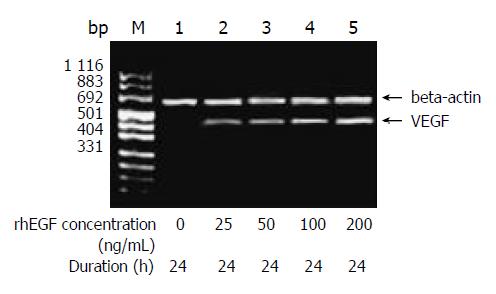
RESULTS: The expression of VEGF mRNA in HepG2 cells cultured in serum-free medium was 0.117. However, 100 μmol/L cobalt chloride for 24 h increased the expression of VEGF mRNA and VEGF mRNA increased gradually with the increase of the concentration and duration of cobalt chloride. Also, 25 ng/mL recombinant human EGF stimulated the expression of VEGF in HepG2 cells and the expression increased with the increase of EGF concentration. 5 μmol/L LY294002 inhibited the expression of VEGF stimulated by cobalt chloride or recombinant human EGF and the inhibition decreased step by step with increase of the concentration of LY294002. But even 20 μmol/L LY294002 could not completely block the expression of VEGF. In contrast, PD98059 had no inhibitory effects on the transcription of VEGF stimulated by cobalt chloride or recombinant human EGF.
CONCLUSION: The overexpression of VEGF in HCC could be promoted by hypoxia and EGF expression in HCC. The signal transduction pathway of VEGF transcription in HepG2 cells may be through PI3K pathway, but not through p42/p44 MAPK pathway.
- Citation: Huang GW, Yang LY, Lu WQ. Effects of PI3K and p42/p44 MAPK on overexpression of vascular endothelial growth factor in hepatocellular carcinoma. World J Gastroenterol 2004; 10(6): 809-812
- URL: https://www.wjgnet.com/1007-9327/full/v10/i6/809.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i6.809
Hepatocellular carcinoma (HCC) is one of the most common cancers in China. Owing to the improvement of surgical technique and early diagnostic methods, the resection rate of HCC has increased. However, the postoperative relapse rate remains high, which has become one of the main obstacles to the therapy of HCC. The mechanisms leading to the relapse are still unclear. Much effort has been done to make it clear. Recently, neovascularization, commonly observed in HCC, has been suggested to play important roles in the relapse of HCC[1-3]. Vascular endothelial growth factor (VEGF) is one of the most potent proangiogenic agents to date. It is confirmed that there is overexpression of VEGF in HCC tissue. However, the mechanisms of VEGF overexpression in HCC are still unclear.
Phosphatidylinositol 3-kinase (PI3K) is a kind of lipid kinase which generates specific inositol lipids that are implicated in many cellular processes, such as cell growth, proliferation, survival, differentiation and cytoskeletal changes[4,5]. MAP kinases are a family of serine/threonine kinases activated through a signaling pathway triggered by numerous agonists such as growth factors, hormones, lymphokines, extracellular matrix components, tumor promoters and stress factors[6]. It has become clear that MAPKs regulate almost all cellular processes, from gene expression to cell death. Recently, both PI3K and p42/p44MAPK pathways have been both found to play important roles in the regulation of angiogenesis[7-9].
The objective of the current study was to investigate the mechanisms leading to the overexpression of VEGF in HCC in vitro and the possible roles of PI3K and p42/p44 MAPK in the regulation of VEGF transcription in hepatoma cells.
The HepG2 cells (a hepatocellular carcinoma cell line), provided by the Center of Cell Culture in Xiangya Medical College, were cultured in DMEM supplemented with 100 mL/L fetal bovine serum (FBS) and incubated at 37 °C in a 50 mL/L CO2 atmosphere. The cells were cultured overnight in DMEM without FBS before intervention.
Hypoxia-inducer cobalt chloride (Sigma) and recombinant human epidermal growth factor (rhEGF, Promega) were used to stimulate the cells. Before stimulation, LY294002 (Promega) and PD98059 (Promega) were used to pretreat the cells to test the function of PI3K and p42/p44 MAPK, respectively.
Seven groups included: 1. no stimulation group (NS); 2. cobalt chloride group (CC), 100-400 μmol/Lcobalt chloride stimulated the cells for 3-24 h; 3. EGF group (EGF), 25-200 ng/mL rhEGF stimulated the cells for 24 h; 4. cobalt chloride plus LY294002 group (CCL), 5-20 μmol/LLY294002 stimulated the cells 30 min before cobalt chloride treatment; 5. EGF plus LY294002 group(EL), 5-20 μmol/L LY294002 stimulated the cells 30 min before rhEGF stimulation; 6. cobalt chloride plus PD98059 group (CCP), 25-100 μmol/L PD98059 stimulated the cells 30 min before cobalt chloride treatment; 7. EGF plus PD98059 group (EP), 25-100 μmol/L PD98059 stimulated the cells 30 min before rhEGF treatment.
After incubation for a given duration, the cells were harvested and the total RNA was extracted by using the TRIZOL Reagent (GIBCO BRL., USA). One microgram of RNA was reversely transcribed into cDNA in 20 μL reverse transcriptional system containing 50 mmol/L Tris-HCl, 75 mmol/L KCl, 3 mmol/L MgCl2, 0.5 μg oligo-dT primer, 0.5 mmol/L deoxynucleotide triphosphate (dNTP), 20U RNasin and 200 U murine Moloney leukemia virus (M-MLV) reverse transcriptase (Promega Corp., Madison, WI), at 37 °C for 1 h. After reverse transcription, 5 μL of product was added to PCR buffer containing 10 mmol/L Tris-HCl, 1.5 mmol/L MgCl2, 50 mmol/L KCl, 1 g/LTriton-X-100, 0.2 μmol/L forward primer, 0.2 μmol/L reverse primer, 200 μmol/L dNTP and 2.5U DNA polymerase (SANGON, SHANGHAI). The PCR was performed in a DNA thermal cycler (Perkin Elmer, USA) with a program of denaturing at 94 °C for 5 min; denaturing at 94 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 1 min, the amplification was carried out for 30 cycler. The reaction was stopped in a final extension at 72 °C for 5 min. The forward and reverse primers for human VEGF and beta actin were purchased from SANGON (SHANGHAI) and their nucleotide sequences were listed below: VEGF: forward primer: 5’-TTGCTGCTCTACCTCCAC-3’; reverse primer: 5’-AATGCTTTCTCCGCTCTG-3’ beta actin: forward primer: 5’-ACACTGTGCCCATCTAGGAGG-3’; reverse primer: 5’-AGGGGCCGGACTCGTCATACT-3’ The sizes of PCR products were: 417 bp for VEGF, 680 bp for beta actin, respectively.
PCR products were loaded on a 20 g/L agarose gel with ethidium bromide, and band intensity was quantified by photo image analyzer (Stratagene Eagleeye II). The ratio of band intensity of the sample to the internal standard was calculated in the four reactions that contained significant amounts of both sample and standard, which stood for the amount of expression of VEGF mRNA.
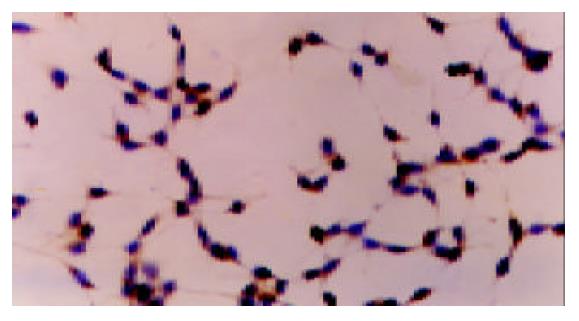
The cells were fixed in cool acetone and sections were stained for VEGF based on streptavidin-biotin-horseradish peroxidase complex formation mentioned before[10]. In brief, the slides were treated with target retrieval solution. Monoclonal anti-VEGF antibody JH121 (200 μg/mL, NEOMARKERS, USA) was used at a dilution of 1:50. The peroxidase reaction was developed using diaminobenzidine and slides were washed. Nuclei were lightly counterstained with hematoxylin. Negative controls were performed using PBS instead of the monoclonal antibody. Two investigators independently evaluated the results of immunocytochemistry.
ANOVA was used appropriately. For the test, a P value of less than 0.05 was considered as significant. All statistics were calculated through SPSS 10.0 software.
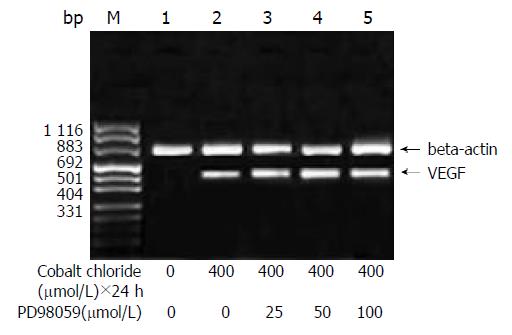
The amount of expression of VEGF mRNA in HepG2 cells cultured in DMEM without FBS was 0.117. With the increase of the concentration of cobalt chloride, the expression of VEGF mRNA increased (P < 0.05) (Figure 1). And also with the increase of the duration of cobalt chloride stimulation, the expression of VEGF mRNA also increased. rhEGF also stimulated the expression of VEGF mRNA in HepG2 cells in a dependant manner of concentration and duration (Figure 2).
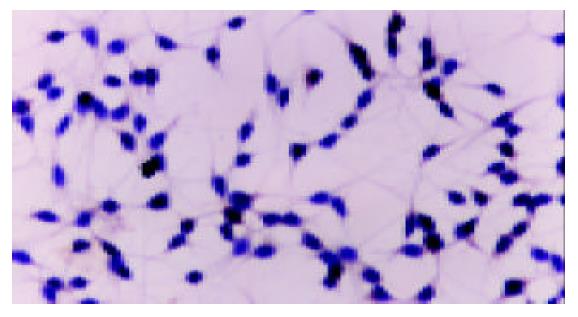
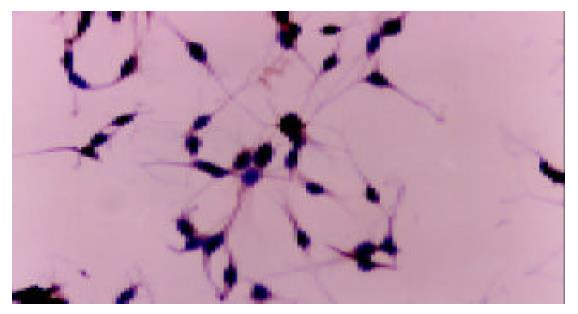
There was no positive staining in HepG2 cells cultured in DMEM without FBS. Cobalt chloride or rhEGF stimulated the expression of VEGF protein in the cytoplasm of HepG2 cells (Figure 3, Figure 4, Figure 5), which demonstrated the results of RT-PCR.
A 5 μmol/L LY294002 inhibited the expression of VEGF stimulated by cobalt chloride or recombinant human EGF and the inhibition decreased step by step with increase of the concentration of LY294002 (P < 0.05). But even 20 μmol/L LY294002 did not completely block the expression of VEGF mRNA (Figure 6). In contrast, PD98059 had no inhibitory effects on the transcription of VEGF stimulated by cobalt chloride or recombinant human EGF (P > 0.05) (Figure 7).
Neovascularization was essential for tumour growth and metastasis[1-3]. The mechanisms underlying the neovascularization in malignancies have been the “hot spot” in the cancer research. VEGF is one of the growth factors proven to be specific and critical for blood vessel formation. In clinical experiment, we have demonstrated that there is VEGF overexpression in HCC and the level of VEGF expression in HCC is correlated not only with microvessel invasion of cancer cells, but also with the survival[11]. So it is very important to make clear the mechanisms and signal transduction pathways that control the VEGF expression in HCC. This would help us find new targets to prevent the neovascularization in HCC so as to preclude the relapse and metastasis of HCC.
Hypoxia, one of the fundamental characteristics of the tumour microenvironment, was demonstrated to be involved in the progression and metastasis of malignancy[12-16]. In the current study, we have shown that hypoxia-inducer cobalt chloride could induce the expression of VEGF in HepG2 cells in a concentration and duration-dependant manner. We have also shown that rhEGF could stimulate the expression of VEGF in HepG2 cells in the same manner. As a result, we could draw a conclusion that both hypoxia and EGF might be the fundamental stimulators of VEGF overexpression in HepG2 cells.
Phosphatidylinositol 3-kinase (PI3K) was a kind of lipid kinase[17-21]. The lipid product of PI3K, phosphatidylinositol-3,4,5-trisphosphate (PIP3), recruited a subset of signaling proteins with pleckstrin homology (PH) domains to the membrane, where they were activated. These proteins included protein serine-threonine kinases (Akt and PDK1), protein tyrosine kinases (Tec family), exchange factors for GTP-binding proteins (Grp1 and Rac exchange factors), and adaptor proteins (GAB-1). Ultimately, these proteins initiated complex sets of events that controlled protein synthesis, actin polymerization, cell survival, and cell cycle entry.
Recently, PI3K has been demonstrated to be involved in the regulation of the transcription of VEGF in certain types of cells. Overexpression of VEGF mRNA has been found in endothelial cells in which the PI3K pathway has been activated[22]. LY294002, the specific inhibitor of PI3K, could inhibit the expression of VEGF in endothelial cells. In addition, PI3K was found to be involved in the regulation of VEGF transcription in cancer cells. In the prostate cancer cell line, LY294002 could completely block the expression of VEGF[23]. Maity et al[24] also demonstrated PI3K was essential in the regulation of VEGF transcription in glioma cell line U87MG. In the current study, we have shown that LY294002 inhibit VEGF transcription stimulated by cobalt chloride or rhEGF in a concentration and duration dependant manner. To our knowledge we demonstrated for the first time that PI3K was involved in the regulation of the signal transduction pathway of VEGF transcription in the hepatoma cells. At the same time, we observed that even 20 μmol/L LY294002 could not block the VEGF mRNA expression completely, which was likened to the results of Zhong[23] and Maity[24]. So there was PI3K-independent pathway in the regulation of VEGF transcription in HepG2 cells.
MAPKs were important signal transducing enzymes, unique to eukaryotes, that were involved in many facets of cellular regulation, such as gene expression, cellular proliferation and programmed cell death[25-27]. Recently, MAPKs has been shown to be involved in the regulation of neovascularization. Rak et al[28] have shown that in the Ras-transforming mouse fibroblast cell line 3T3RAS, VEGF expression was not blocked by LY294002, but by PD98059, the specific inhibitor of p42/p44 MAPK. This result suggested that p42/p44 MAPK was involved in the regulation of VEGF transcription in 3T3RAS cells. In the current study, we did not observe PD98059 could preclude VEGF transcription in HepG2 cells, which indicated p42/p44 MAPK was not involved in the regulation of VEGF transcription in HepG2 cells.
In conclusion, hypoxia and EGF were two stimulators to the VEGF overexpression in hepatoma cells. VEGF transcription might be regulated by PI3K pathway and other PI3K-independent pathway, but not by p42/p44 MAPK pathway.
Edited by Hu DK and Xu FM
| 1. | Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1649] [Cited by in RCA: 1574] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 2. | Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6437] [Cited by in RCA: 6482] [Article Influence: 259.3] [Reference Citation Analysis (0)] |
| 3. | Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2741] [Cited by in RCA: 2688] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 4. | Sotsios Y, Ward SG. Phosphoinositide 3-kinase: a key biochemical signal for cell migration in response to chemokines. Immunol Rev. 2000;177:217-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Roymans D, Slegers H. Phosphatidylinositol 3-kinases in tumor progression. Eur J Biochem. 2001;268:487-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 137] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3924] [Cited by in RCA: 4007] [Article Influence: 167.0] [Reference Citation Analysis (33)] |
| 7. | Dimmeler S, Zeiher AM. Akt takes center stage in angiogenesis signaling. Circ Res. 2000;86:4-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 198] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci USA. 2000;97:1749-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 421] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 9. | Berra E, Milanini J, Richard DE, Le Gall M, Viñals F, Gothié E, Roux D, Pagès G, Pouysségur J. Signaling angiogenesis via p42/p44 MAP kinase and hypoxia. Biochem Pharmacol. 2000;60:1171-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Huang GW, Yang LY. Metallothionein expression in hepatocellular carcinoma. World J Gastroenterol. 2002;8:650-653. [PubMed] |
| 11. | Huang GW, Yang LY, Lu XS, Liu HL, Yang JQ, Yang ZL. Expres-sion of hypoxia-inducible factor 1 alpha in hepatocellular carci-noma and the impact on neovascularization. Zhonghua Xiaohua Zazhi. 2002;22:627-628. |
| 12. | Huang GW, Yang LY. Molecular mechanisms of hypoxia-induced malignant transformation. Shijie Huaren Xiaohua Zazhi. 2001;9:1300-1304. |
| 13. | Huang GW, Yang LY. Hypoxic signal transduction pathway in malignancy. Shijie Huaren Xiaohua Zazhi. 2002;10:1441-1444. |
| 14. | Sutherland RM. Tumor hypoxia and gene expression--implications for malignant progression and therapy. Acta Oncol. 1998;37:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Dachs GU, Tozer GM. Hypoxia modulated gene expression: angiogenesis, metastasis and therapeutic exploitation. Eur J Cancer. 2000;36:1649-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 178] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075-7083. [PubMed] |
| 17. | Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4101] [Cited by in RCA: 4273] [Article Influence: 185.8] [Reference Citation Analysis (0)] |
| 18. | Dhawan P, Singh AB, Ellis DL, Richmond A. Constitutive activation of Akt/protein kinase B in melanoma leads to up-regulation of nuclear factor-kappaB and tumor progression. Cancer Res. 2002;62:7335-7342. [PubMed] |
| 19. | Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Sonenshein GE. Protein kinase CK2 promotes aberrant activation of nuclear factor-kappaB, transformed phenotype, and survival of breast cancer cells. Cancer Res. 2002;62:6770-6778. [PubMed] |
| 20. | Narita Y, Nagane M, Mishima K, Huang HJ, Furnari FB, Cavenee WK. Mutant epidermal growth factor receptor signaling down-regulates p27 through activation of the phosphatidylinositol 3-kinase/Akt pathway in glioblastomas. Cancer Res. 2002;62:6764-6769. [PubMed] |
| 21. | Neri LM, Borgatti P, Capitani S, Martelli AM. The nuclear phosphoinositide 3-kinase/AKT pathway: a new second messenger system. Biochim Biophys Acta. 2002;1584:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Wang D, Huang HJ, Kazlauskas A, Cavenee WK. Induction of vascular endothelial growth factor expression in endothelial cells by platelet-derived growth factor through the activation of phosphatidylinositol 3-kinase. Cancer Res. 1999;59:1464-1472. [PubMed] |
| 23. | Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541-1545. [PubMed] |
| 24. | Maity A, Pore N, Lee J, Solomon D, O'Rourke DM. Epidermal growth factor receptor transcriptionally up-regulates vascular endothelial growth factor expression in human glioblastoma cells via a pathway involving phosphatidylinositol 3'-kinase and distinct from that induced by hypoxia. Cancer Res. 2000;60:5879-5886. [PubMed] |
| 25. | Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3062] [Cited by in RCA: 3304] [Article Influence: 143.7] [Reference Citation Analysis (0)] |
| 26. | Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1322] [Cited by in RCA: 1397] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 27. | Ling MT, Wang X, Ouyang XS, Lee TK, Fan TY, Xu K, Tsao SW, Wong YC. Activation of MAPK signaling pathway is essential for Id-1 induced serum independent prostate cancer cell growth. Oncogene. 2002;21:8498-8505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Rak J, Mitsuhashi Y, Sheehan C, Tamir A, Viloria-Petit A, Filmus J, Mansour SJ, Ahn NG, Kerbel RS. Oncogenes and tumor angiogenesis: differential modes of vascular endothelial growth factor up-regulation in ras-transformed epithelial cells and fibroblasts. Cancer Res. 2000;60:490-498. [PubMed] |