CASE SERIES
A retrospective review performed on 4 patients presenting incomplete colonic obstruction secondary to left-side colon cancer received a colonic stent at our institution between June 2000 and July 2003.
Clinical and radiographic criteria for patient eligibility to this treatment included (a) symptoms of obstruction with constipation for a period longer than 48 h, abdominal distension, nausea, vomiting, or abdominal pain, and (b) conventional radiologic evidence of colon-rectal obstruction (confirmed by abdominal computed tomography).
We excluded patients if they manifested clinical evidence of bowel perforation and free intraperitoneal air on abdominal radiograph, peritonitis, massive gastrointestinal bleeding, a fixed rectal mass.
All patients underwent baseline endoscopic evaluation for delineation of tumor length and demarcation. In all cases, histopathologic findings from biopsy revealed adenocarcinoma. After adequate explanation regarding discouraging complications (including difficulty of insertion because of propagation of the tumor, possible perforation on insertion and expansion of the stent, stent migration and tumor ingrowth), informed consent was given by each patient and family.
Case 1
In July 2000 a 90-year-old man came to our department because of rectal bleeding, appearing 1 mo before, and ileus symptoms. At admission the physical examination demonstrated abdomen distension and a rectal mass on finger exploration. Laboratory tests revealed an anaemic condition alone. American Society of Anesthesiologists (ASA) status was III. Then the patient underwent nasogastric decompression and received intravenous fluid supplements. CT of the abdomen and pelvis showed a rectal mass involving the whole wall thickness. Colonoscopy identified a substenotic rectal mass, measuring 4 cm in length, 8 cm away from the dentate line, which was dilatated with a TTS-balloon (BE-6 OLYMPUS - Europe, Amburg, Germany). We decided to stent the lesion and opted for this kind of temporary treatment because of stent supply times. Balloon dilation showed an improvement of abdominal symptoms with gas and liquid stools transit. In the meantime we supported the patient with a liquid diet and total parenteral nutrition. Small enemas were also administered. On d 10 after admission, we inserted a Wallstent prosthesis (Schneider, Bulach, Switzerland) measuring 9 cm in length and 22 mm in diameter. The patient’s symptoms improved immediately after stent placement with passage of stools and flatus from the anus. He died 6 mo after stent placement due to progression of the initial disease without constipation symptoms or signs.
Case 2
In July 2002 a 75-year-old man was admitted complaining of abdominal pain and constipation for 3 d. Family history was positive for familiarity with neoplastic colonic disease (one sister had died). Past history: in 1984 radical cystectomy with urostomy for bladder carcinoma, chronic renal failure, in 1994 surgical clearing for choledocholithiasis, cerebral ischaemia and outcomes of lacunar encephalopathy, left hemiparesis, sclero-hypertensive cardiomyopathy, ventricular extrasystoles. In the last 6 mo, a body mass loss of 10 kg and poor appetite were noted. The patient had also been suffering from hypogastric pain for a month. For this reason, on 27 of July 2002 he underwent colonoscopy that revealed a circular stenosis 75 cm away from the anal verge, in the distal transverse colon (near splenic flexure) line, and unable to get through. Biopsy of this tissue demonstrated a histopathological finding of a well-differentiated adenocarcinoma. On August 28, 2002 he was referred to our institution because of ileus symptoms.
At physical examination, his abdomen was markedly distended. Laboratory tests disclosed hyper blood urea and hyper-creatinine level, malnutrition with a total protein level of 5.4 g/dL (normal value 6.4-8.3 g/dL), a white blood cell count of 8.500 mm3 (with normal differential cell and platelet counts), a hemoglobin level of 12 g/dL, a hematocrit of 36%. The ASA status was IV.
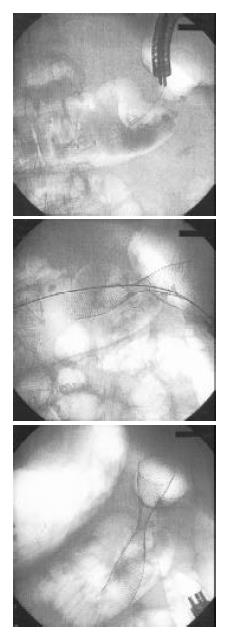
Plain abdominal Rx at admission showed fluid line. He was then subjected to total parenteral nutrition and a nasogastric tube to reduce gastrointestinal pressure. On the following days, ileus was temporally improved, on September 4, he underwent definitive colonic decompression using a Wallstent (Boston Scientific Microvasive, Minnesota, USA) measuring 9 cm in length and 22 mm in diameter (Figure 1). After one year he is still alive, follow-up control was scheduled for the 30th 2003.
Figure 1 Radiologic sequence of stenting procedure in left transverse colon.
Case 3
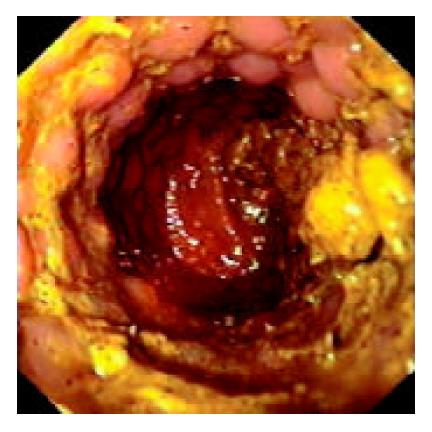
An 85-year-old woman was admitted to our department in October 2002 suffering from rectal bleeding first noted the year before. On admission the patient was diagnosed with ileus symptoms due to ulcered, exophytic substenotic recto-sigmoid neoplasm, 5 cm long starting 14 cm from the anal verge as a result of colonoscopic examination. Laboratory studies disclosed malnutrition (total protein level of 5.6 g/dL) and anaemia (hemoglobin level of 10.7 g/dL, hematocrit of 33.5%). ASA status was IV. Plain computed tomography revealed tumor shadow of the sigmoid and the cephalic side of the colon was markedly dilated with fluid collection. Swelling of intraperitoneal lymph nodes and an abundant bilateral pleural effusion were also noted. We inserted a Precision Ultraflex prosthesis (Boston Scientific Microvasive, Minnesota, USA), measuring 9 cm in length and 25 mm in diameter. We obtained immediate recovery of colonic transit (Figure 2). She was discharged after 2 d and died after 45 d.
Figure 2 Endoscopic view of Wallstent positioned in the sig-moid colon with recovery of intestinal transit.
Case 4
A 61-year-old woman, without any medical history, had its beginning in March 2003, when she noted relapsing episodes/ events of sub-occlusion and a body mass loss of 10 kg. At hematologic examinations, carcino-embryonic antigen and carbohydrate antigen 19-9 levels were significantly elevated at 8000 ng/mL and over 10000 U/mL, respectively. Plain computed tomography performed on May 29 showed multiple liver metastases, ascites and peritoneal carcinomatosis. Colonoscopic examination showed a circular stenosis at the sigmoid. Histopathological findings demonstrated adenocarcinoma. Initial support therapy was sought at a medical institute and then she was referred to ours for the required treatment. ASA-status was IV. A water-soluble contrast-media enema performed just after the colonoscope introduction, demonstrated a 3 cm long stenosis. A Precision Ultraflex stent, measuring 9 cm in length and 25 mm in diameter, was selected and positioned. The day after stent insertion, we saw a full canalization. The patient was discharged on the 3rd hospital day and she is still alive (end of follow-up: August 2003).
Before stent placement, all the patients underwent one or more colonic enemas, depending on the site of obstruction for cleaning the colon below the stricture. The patients were placed in the supine position only using a suitable combination of intravenous medication appropriate for a colonoscopic procedure as sedation and analgesia. There was no routine administration of antibiotics. Vital signs (pulse rate and oxygen saturation) were monitored continuously.
As regards the stent placement technique used, once the colonoscope was inserted, a water-soluble contrast media was injected for careful assessment of the length and the morphology of the stricture by fluoroscopy. Then, an Amplatz superstiff guidewire (Boston Scientific Microvasive, Minnesota, USA) was inserted through the colonoscope channel and advanced as far as possible into the proximal bowel, and kept in situ. The instrument was then removed and reinserted adjacent to the wire. The stent was then deployed over the guidewire and across the stenosis. Using fluoroscopy, the stent was placed with each end equidistant from the tumor margins except for the first case. Deployment was performed under fluoroscopic and endoscopic guidance. Finally, endoscopic and X-ray images were used to assess the accuracy of the stent position.
Dedicated enteral stents were used (Enteral Wallstent/ Precision Ultraflex endoprostheses). We chose enteral stents about 5 cm longer than the stricture, 1 or 2 cm of the stent to extend beyond both ends of the tumor.
We did not dilate the strictures before stent placement, except for the first patient, because neoplasms were not totally obstructing, but it should be remembered that this procedure could increase the risk of colonic perforation.
To determine the stent position and the relief of colonic obstruction until the patients were discharged, a conventional radiograph of the abdomen was obtained and changes in bowel gas patterns were analyzed within 24 h of stent placement and each subsequent day thereafter.
All patients tolerated the procedures well, which were technically successful.
There were no immediate technical complications associated with stent placement, and no perforation, major bleeding, or death related to endoscopic procedure. Each of the patient’s symptoms improved immediately after stent placement with passage of stools and flatus from the anus. Initially the patients were able to take at least liquids, later they assumed a low-residue diet. The only delayed complication was dislodgment of 1 stent (25%) after one month.
The average survival time was 22.5 wk, and ranged from 6 to 48 wk. Two patients were alive at the end of the study period, two died because of the natural disease history. No patients showed clinical symptoms of obstruction at the time of death or termination of the study, all tolerated oral semi-solid feedings.
DISCUSSION
Malignancy is the major cause (85%) of acute colonic obstruction (ACO) and 10% - 30% of patients with colonic cancer had a large-bowel intestinal occlusion at presentation[1-3].
Therefore, not surprisingly, ACO is considered as a surgical emergency, traditionally treated with surgical intervention that, though effective, was associated with high morbidity (10% - 36%) and mortality (6% - 30%) rates[4].
Emergency surgery, mainly in acutely ill patients, often results in a diverting colostomy alone, which may be associated with a morbidity rate of 20% - 40% and a mortality rate of more than 10%[5].
Indeed, many of these patients have already reached an advanced stage of the disease at the time of diagnosis. For patients with advanced tumors no longer resectable, widespread metastatic disease, peritoneal carcinomatosis, unfortunately the only therapeutic options have been palliative, with colostomy being the only reasonable and often unavoidable surgical option[6]. Besides, many of these patients were elderly (up to 50% between 70 and 89 years old) and instable because of significant co-morbidities, all this made them of high surgical risk[7-9]. Moreover this treatment option entailed a significant decrease in quality of life, with major psychological repercussions[10]. In addition, this unfortunate group of patients had short life expectancies (mean survival of patients who had hepatic metastases at the time of surgery was only 4.5 mo)[11] and it was of paramount importance to keep them out of hospital. So, it is therefore preferable to seek more comfortable therapeutic approaches.
The primary goal of a non-surgical approach for treating ACO is to avoid the need of emergency surgical treatment in non stabilized patients. Such non-surgical alternatives to colostomy as balloon dilation[12] and ablative methods (cryotherapy, electrocoagulation, laser photocoagulation)[13,14], have been used in inoperable patients or with unresectable tumors. Laser therapy (LT) has gained considerable support by virtue of reports of its high initial success in luminal diameter increasing. Nd:Yag laser therapy was considered the treatment of choice for endoscopic palliation of advanced rectal carcinoma. In 1986 Mathus-Vliegen reported a success rate of 85% - 95% (5, 15). The drawbacks of LT were expensive equipment, time consuming sessions, lack of immediate relief of symptoms, need for repeated sessions to maintain patency, needs to repeat sessions, even every 5 to 9 wk[12,16,17], significant (13%)[18,19] risk of major complications like stenosis, perforation[20], fistula (3.2%), abscess (1.7%) and bleeding (4.1%), as published by Gevers et al[21].
In conclusion, LT is affected by such shortcomings as the need to be repeated periodically to maintain patency and limited applicability restricted solely to selected patients[22] with tumors in distal locations.
In recent years, enteral stenting has emerged as an effective alternative to the surgical approach. However, the concept of colonic decompression using “stent” in obstructing colonic tumors, in the absence of peritonitis[23], is not new. Initial reports were published in the early 1990s with some promising results but containing only a handful of patients, Lelcuk in 1986[24] and Keen[25] in 1992 passed a nasogastric tube respectively through the tumor to relieve obstruction. In 1991 the first report describing use of a metal stent in the rectum was published by Dohmoto [26]. The following year, in a small series of four and two patients respectively, Spinelli in 1992[27] and Itabashi in 1993[28] confirmed the feasibility of inserting self-expanding metal stents for immediate relief of acute colonic obstruction due to rectal malignant tumors. In 1998 De Gregorio[29] published a large multicenter retrospective study (24 pts) evaluating the success of the placement of colorectal stents for palliation, reporting a 100% technical success and a 96% clinical improvement. Using various kinds of stent, in 25 patients, Baron’s group[30] had less impressive results. They failed to put in the stents in 6% of their patients because of technical problems and only 85% of their stented patients had relief from obstruction.
The question as to which strictures by site are amenable to colonic endolumenal stenting (CELS) is not well addressed in literature by many authors. Similarly their description of the sites of lesions they have stented is unclear. The most commonly reported cases of CELS in literature were of lesions within the rectum and rectosigmoid, which was unsurprising as 70% of the obstructing colonic strictures were located in the left colon[1,31].
While the initial series excluded patients thought to have lesions located in difficult anatomical positions[32]. To date location has not appeared to be a limitation for CELS[30,33,34], neither did the length of the tumor. If the lesion was not successfully or totally covered by the stent, an additional stent could be placed.
Distal extent of disease does not limit suitability for stenting, although lesions less than 5 cm from the anal verge (dentate line) might be inappropriate[35,36] and difficult to palliate with a metal stent, because of perianal trauma occurring due to stent irritation[37]. Despite the recent advances in stent technology, the search for the ideal enteral stent has not stopped. Enteral stents should be flexible enough to allow placement but should remain in position once deployed[38].
Initially since dedicated colonic stents were not available, a variety of stents originally designed for use elsewhere were used, as reported in literature studies, including the preferred enteral Wallstent®[39-41]. Others were Endocoil® (Euromed Inc), esophageal nitinol Strecker stent, Gianturco self-expanding Z-stent, Ultraflex®, Instent Esophacoil®[28,30,34,39,40,42-45]. There was no noticeable difference in the outcomes using any of the above stents.
Metallic stents are of two types, expandable or self-expanding (so-called self-expanding metal stents, or SEMS). Because of their flexibility SEMS are easier to deploy than rigid tubes and allow peristalsis to continue, but they usually have a narrow lumen and are prone to tumor ingrowth. Some of these mesh type stents have been coated with polyurethane or other materials to prevent occlusion by tumor although this may increase the likelihood of dislocation. Covered stents have the advantage of resisting tumor ingrowth but tend to be less stable and more rigid, they require a larger delivery system, and are more likely to migrate. They are thus more difficult to deploy at distant locations through a tortuous delivery path[46]. Uncovered stents are more flexible, and at least one can be passed through the working channel of an endoscope. However, when used for long-term palliation of malignant obstruction, they are subjected to tumor ingrowth and resultant obstruction. Most authors now use SEMS made of nitinol, a nickel/titanium alloy, which has <<shape memory>> meaning that once deployed, it adopts a preformed shape, an advantage over expandable systems that require dilation. Stent technology is currently evolving rapidly, and devices are now being developed specifically for colorectal applications.
The mean technical success, defined as successful stent placement and deployment, ranged from 64% (40) and 100%[32,28,39,47]. Failure to stent deployment was usually a result of inability to pass a guide wire through a lesion, other reasons for technical failure, apart from tight or tortuous stenosis through which the guide wire could not be passed[30,34,40,44,45] included insufficient length of the stent to span the entire stricture, inadequate introducer lengths[30,44], floppy introducer system and incorrect deployment of the stent (42). A higher rate of successful stent placement could be achieved with more distal lesions. The most proximally placed stents described in literature till now have been in the right and proximal transverse colon[30,34].
The stent placement procedure is generally painless and neither anesthesia nor analgesia is provided. It was frequently sufficient to provide a suitable combination of intravenous medication for a simple colonoscopy procedure[9,17]. Clinical success, that is relief of obstructive symptoms, defined as colonic decompression within 96 h without endoscopic or surgical reintervention after stent placement (this time interval was chosen because it was integral to the definition used in nearly all the papers reviewed) has been reported in 75% - 96%[2,23,30].
Stent placement complications were common to many authors, distinguishing the early from late ones. In previously published series[9,29,34,45,48] the complication rate has been reported to range from 14% to 42%, most complication being minor. In the literature surveyed these were successfully managed with medical or supportive treatment in the majority of cases. Less seen complications included minor rectal bleeding, anorectal pain, temporary incontinence (11%)[42], fecal impaction (8%)[40] and severe tenesmus. Rectal bleeding (0% - 100%) was usually hemodynamically insignificant and self-limited. It could result from mucosal irritation, pressure necrosis of the stent in the colonic mucosa, or friability of the tumor itself [49]. Anorectal pain (5% - 100%) might occur and was usually mild and transient, lasting for only 3-5 days, it was easily controlled by analgesia[39,45,49]. Severe tenesmus occurred during the first 48 hours and might be controlled with non-steroid anti-inflammatory drugs. It seemed to be related to insertion of the stent in a lower portion of the rectum[50]. However, Camuñez[51] described a patient who was readmitted twice because of persistent analgesic therapy, and was offered a colostomy which ultimately was refused.
Stent migration, restenosis, and perforation were the major complications encountered with colonic stent placement. Stent dislocation (0% - 44%) and obstruction (0% - 33%) were reported as the most common major complications described, but are not usually serious. Stent migration has been reported to occur in as many as 40% of cases and was usually detected on follow-up radiographs within 1 week of insertion. Camuñez[22] believed the cause of stent migration was shrinkage of the tumours as a result of adjuvant chemotherapy. Generally, it appeared that predisposing factors included inappropriate stent selection as covered stent or those with too narrow diameter (with weak radial expansive strength), colonic angulation and post-operative chemotherapy or radiation therapy[38,49,52]. Consequently, specific monitoring to check for possible reduction in tumor size and assess the advisability of stent extraction, is recommendable in patients receiving adjuvant therapy. When stent migration occurred, the stent might generally be passed spontaneously or require endoscopic removal and redeployment[49]. Some stents which became dislocated and were expelled did not necessarily require replacement, since bowel function was adequately maintained.
Restenosis has been reported in up to 25% of cases and was usually due to malpositioning of the stent, as well as impaction with stool or food matter (it has been recommended that patients with colorectal stent ingest a low-residue diet and use stool softeners to lessen the likelihood of stent obstruction)[38,53] but especially tumor ingrowth. Stent obstruction was usually amenable to a variety of nonoperative measures such as further coaxial restenting[30] and laser therapy[42]. However it would be interesting to point out that in Dohmoto’ study 15% of patients needed a palliative colostomy to treat stent occlusion related to tumor ingrowth.
Restenosis due to tumor overgrowth (extension of tumor above or below the stent) could also be treated with a second stent. It could be best prevented by deploying the original stent 2-3 cm above or below the lesion when possible.
The most serious and potentially devastating complication of colonic stent placement was colonic perforation, reported in 0% - 16% of cases[49,54,55]. Perforation can be either early or late, it should be suspected in patients who complain of abdominal pain during or immediately after the stenting procedure. Colonic perforation may have different causes, related either to the method itself or to the stent. One of the major causes is excessive balloon dilation of the stricture, causing full-thickness tear of the mucosa. Baron et al[30] reported colonic perforation in four patients, associated with balloon dilation of the stricture at the time of stent placement in three of the patients. Without balloon dilation, perforation rate might fall below 5%. Therefore, this practice is no longer recommended[56], and stents are allowed to slowly self-expand.
Fortunately, it is possible to achieve stent placement even in patients with tight strictures without employing dilatation when modern stents are used. In addition, as perforation is more likely to occur during guidewire manipulation, the introduction of a wide range of soft-tipped guide-wires may contribute further to prevention of perforation. In the experience of many authors[34,44,22] perforation due to manipulation of guidewires and catheters has been asymptomatic, and it has been possible to complete the procedure in all cases. Late perforation could occur (though it is rare) due to pressure necrosis and erosion through the colon. Histologically, the side of the cancerous lesion compressed by the stent was thin and consisted of a serosal layer alone. Granulomatous change was detected histologically in normal mucosa that was in contact with the metallic stent. These histological changes were caused by direct compression or ischemic damage due to the self-expanding stent. Therefore, if SEMS is inserted in patients with complete obstruction due to advanced carcinoma infiltrating the serosa, Kusayanagi’s experience suggests that a risk of perforation might develop approximately 2 months later[49,57]. Several authors concluded that the possibility of colonic perforation as a potential complication must be kept constantly in mind and that consequently close clinical observation was required particularly in the days immediately following stent placement[22].
Because of the continual changes in design, it is difficult to compare complication rates among different kinds of stent. However, some general conclusion may be drawn. The decreasing diameters of delivery systems make perforation a rare occurrence, and it is generally related to pre-stenting treatments.
Indeed, certain authors, prior to stent placement, pretreated the neoplastic strictures endoscopically first to canalize an obstruction using laserltherapy[17,48], argonplasma or coagulation, others used mechanical dilatation with endoluminal balloon catheters[30,41]. Nevertheless, the added efficacy of these approaches is unproven, and certainly they increase the risk.
Patients stented for palliation usually underwent clinical follow-up, conventional abdominal radiographs were obtained at monthly intervals. Period of patency was defined as the period from stent placement to the recurrence of symptoms of obstruction in clinically successful cases[58]. No absolute data exist on the mean time to occlusion of stents, also because this procedure has been carried out in a small total number of patients in the same short follow-up periods. Hence, assessment of stent patency and long-term behavior are difficult to define and data coming from literature are wide-ranging. Thus, according to Baron[30], stent duration ranged from 2 to 64 wk (mean 17.3 wk). Kusayanagi’s data[57] was also in this range. According to him the mean time before re-obstruction was approximately 10 wk and the median follow-up for Repici[59] was 21 wk (range 1-46). Moreover, no recurrence of obstruction was observed during the follow-up period. For Camuñez[51] follow-up lasted an average of 138 ± 93 d (range: 36-334 d), and the estimated primary stent patency rate was 91% at 3 and 6 mo. Even for mortality rate existing data in literature are controversial. For some authors[17,60] no deaths have been reported directly attributable to the stenting procedure, on the contrary, Spinelli[48] had a mortality rate of 3%.
Until now we have seen that, when LBO due to cancer has progressed beyond the stage of any curable intervention, there are only two options to relieve the obstruction by colostomy or by use of a stent, since both are capable of relieving acute obstruction and stenting clearly offers a preferable quality of life. Another important issue to consider is their cost, considering the fact that the principal cost determinants for the stenting option was the cost of the stent itself and the duration of in-hospital treatment[61,62]. Few publications examined the cost impact of stenting compared to surgical decompression. One study[34], concerning the cost of the stenting procedure compared with a control group undergoing only surgical treatment for malignant colorectal obstruction, showed that the overall cost of treating the stented group was 19.7% lower. According to Zollikofer’s data [63], a British study[64], comparing the cost of management by stenting 16 patients with acute large bowel obstruction with 10 unselected patients previously managed by surgical decompression, estimated that the cost of a palliative care was less than half of that of a surgically decompressed case. These cost savings were due mainly to the shorter hospital stay associated with stenting. Other factors were fewer surgical procedures, reduced operating time and fewer days in intensive care room. On the other hand, these data have much more relevance considering that the often hidden cost of stoma care in the community cannot be overlooked, about Euro 100 per mo is required for disposable stoma bags for each patient.