INTRODUCTION
Isolated and cultured hepatocytes in vitro are an important tool for hepatic disease study, and have been extensively used in basic researches of liver disease, pathophysiology, pharmacology and other related subjects[1-5]. Furthermore, they are a core material of bioartificial liver system which has been developed rapidly in recent years[6-10]. However, normal culture condition of hepatocytes in vitro differs greatly from the environment in vivo, and is difficult to maintain the physiological function of hepatocytes, leading to restriction of their extensive uses. Along with the advancs in the research of bioartificial liver support system and its related fields, a highly active hepatocyte culture system is urgently required to meet the quality requirement of cultured hepatoctyes in these studies[11-15].
It has been discovered that collagen is one kind of important matrix of hepatocytic basal membrane. Type I rat tail collagen is usually used as a coated material for hepatocyte culture. It promotes attachment and growth of hepatocytes. The effect of culture is much better than that without collagen coating, indicating that collagen matrix might be an important condition for long-term hepatocyte culture[16-18]. Based on this idea, we performed the collagen mixture culture for rat hepatocytes and the collagen sandwich culture for new-born Chinese experimental piglet hepatocytes, in order to improve the effect of hepatocyte culture, and provide experimental data for bioartificial liver.
MATERIALS AND METHODS
Animal
Wistar rats (both sexes, less than one month old) and newborn Chinese experimental piglets (both sexes, one week old) were provided by the Experimental Animal Center, Third Military Medical University. All studies were performed with the approval of Experimental Animal Committee in our university. The animals were fed under standard conditions and fasted for 4 h before the experiment.
Reagents
Type IV collagenase, type I mouse collagen (collagen VII) and epidermal growth factor (EGF) were purchased from Sigma. DMEM medium, RPMI 1640 medium and FBS were from Gibco. Acrylamide, N,N’-methylene-bis-acrylamide, sodium dodecyl sulfate (SDS), Coomasie blue were from Shengong Biothech Company (Shanghai, China).
Cell isolation
Hepatocytes were harvested using a two-step collagenase perfusion technique described previously[19,20]. Breifly, an animal was anesthetized by pentobarbital sodium (30 mg/kg), followed by sterilizing the skin, opening the abdomen, exposing the portal vein, and heparin sodium (100-150 u) was injected into the portal vein. When the liver was isolated at liver hilus, it was first perfused with calcium and magnesium-free Hank’s buffer at 80-100 mL/min for 10-15 min. The liver was then perfused with 0.5 g/L collagenase solution at 50-70 mL/min for 10 min. The two perfusion systems were kept at 37°C - 38 °C. After perfusion, the liver capsule was incised. The thick fibrous connective tissue was discarded, and cell suspensions were harvested. The cell suspensions were further digested at 37 °C for 10-15 min in case. When RPMI 1640 medium was used for cessation of digestion, the released cells were filtered through three-layer sterilized gauze and washed via three centrifugations (50 g). The hepatocyte viability greater than 95% as determined by trypan blue exclusion was used for culture.
Collagen mixture gel culture
The collagen mixture consisted of a 4:1 ratio of rats tail collagen and 4× DMEM brought to a pH of 7.2 using 1mol/L NaOH, 1 × 106 isolated rat hepatocytes were mixed with collagen solution and plated in a flask. The height of hepatocyte-collagen mixture was 0.3-0.4 cm. The mixture was incubated in a 37 °C incubator for 2 h until the collagen mixture was gelled, resulting in hepatocyte-collagen mixture gel. After washed 2-3 times with PBS, RPMI 1640 medium containing 100mL/L FBS and EFG was added and the cells were cultured in normal conditions. The medium was changed daily and the culture was over on day 9. The normal single layer culture was used as control, the seeding density and medium were the same as collagen gel mixture culture.
Collagen sandwich culture
The collagen solution was prepared as described above, and coated on a 6-well plate. Collagen solution (1.2 mL) was added into each well and incubated in a 37 °C incubator for 2 h until the collagen was gelled. Freshly isolated hepatocytes (106) were plated on a 6-well plate precoated with collagen gel and cultured with serum free RPMI 1640. After 24 h, the culture medium was replaced by 1.2 mL/well collagen solution, and incubated in an incubator for 1 h until the collagen was fixed. The hepatocytes were cultured under the above conditions. The medium was changed daily and the culture was over on day 7.
Morphological and functional observations of cultured hepatocytes
Supernatant of the medium was collected every day during the rat hepatoclytes-collagen gel culture, and BUN was measured using a biochemical autoanalyzer (Model 7020, Hitach Co., Tokyo, Japan). Twenty-five µl of the supernatant of serum free medium with piglet hepatocyte sandwich culture was harvested on days 1, 2, 5, and 7 respectively, and separated on a 120 g/L SDS-PAGE, resulting in porcine albumin bands. The gel was stained with 5 g/L Coomasie blue, and the images were analyzed with the gel documentation-analyzing systems (Gel DocTM 2000, Bio-Rad, USA). LDH-release in the supernatants of the two kinds of hepatocyte culture media was analyzed by ultraviolet dynamic method. Morphological changes of hepatocyes in both collagen gel mixture and sandwich cultures were observed under a phase-contrast microscope.
RESULTS
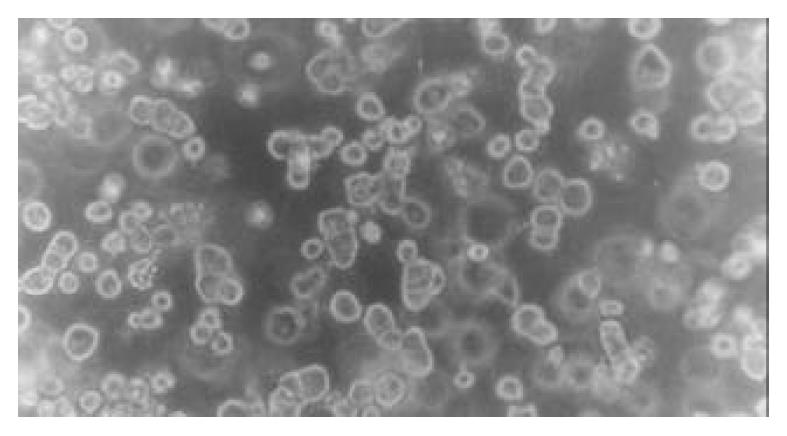
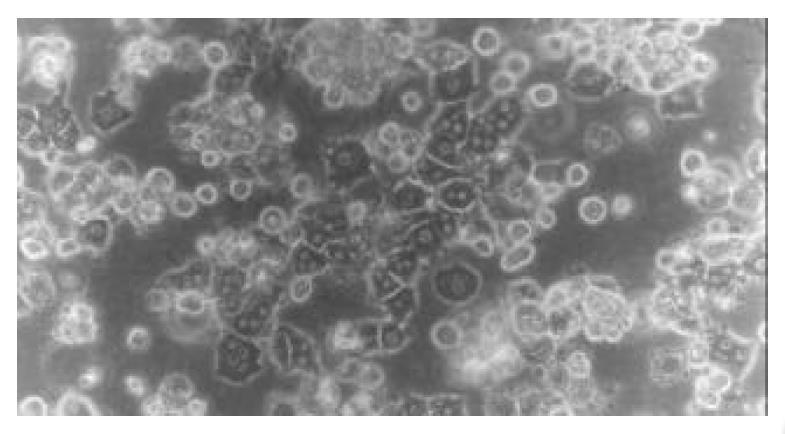
Morphology of rat hepatocytes cultured in collagen gel mixture When freshly isolated hepatocytes were mixed with collagen and seeded on the culture plate, spherical hepatocytes were mixed in a slightly transparent collagen solution. When the collagen solution was gelled, the heptocytes were uniformly fixed within. After cultured for 24 h, most hepatocytes remained spherical in shape with part of the cells transformed into polygonal shape. The majority of hepatocytes adhered to each other presenting a fascicular growth (Figure 1). After cultured for 48 h, the majority of hepatocytes reconstructed their cellular polarity presenting a typical and even polygonal morphology. Binuclei were observed in the majority of hepatocytes, the hepatic plate structure was gradually formed, and the boundary between hepatocytes was perfectly clear and bright, illustrating the formation and participation of bile canaliculi (Figure 2). Such cytomorphological characteristics remained till the end of culture. The rat hepatocytes via an ordinary single layer culture quickly sank and attached to the bottom of culture plate after seeding. Their cytomorphology changed from freshly isolated spherical morphology to a monolayer flat polygon. The cells became flat 48-72 h later and gradually lost their typical polygonal characteristic, and eventually, the hepatocytes were in conjunction with each other and grew in patches.
Figure 1 Morphology of rat hepatocytes in collagen gel mix-ture culture at 24 h (phase-contrast microscope ×200).
Figure 2 Morphology of rat hepatocytes in collagen gel mix-ture culture at 72 h (phase-contrast microscope ×200).
Morphological changes of hepatocytes cultured in sandwich configurations
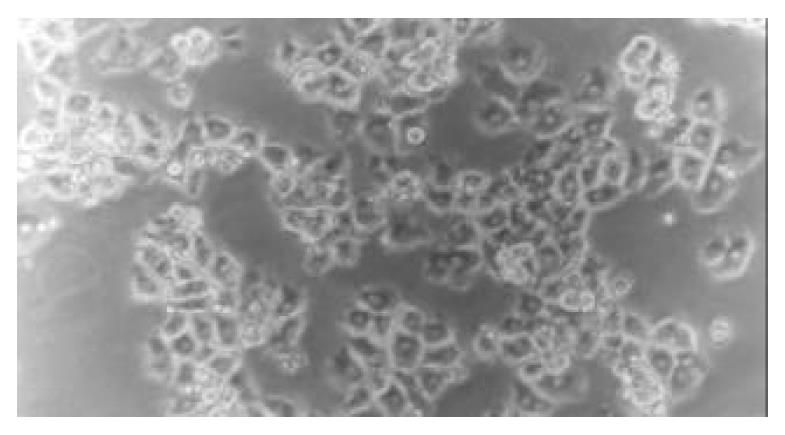
After freshly isolated piglet hepatocytes were seeded on a single layer of collagen gel, the hepatocytes soon attached to the culture plates and their spherical morphology changed quickly to polygonal morphology. After 4 h of culture, the hepatoctyes exhibited colonial and fascicular growth, showing typical, uniform polygonal morphology (Figure 3).
Figure 3 Morphology of piglet hepatocytes cultured on a single layer of collagen gel at 4 h (phase-contrast microscope ×200).
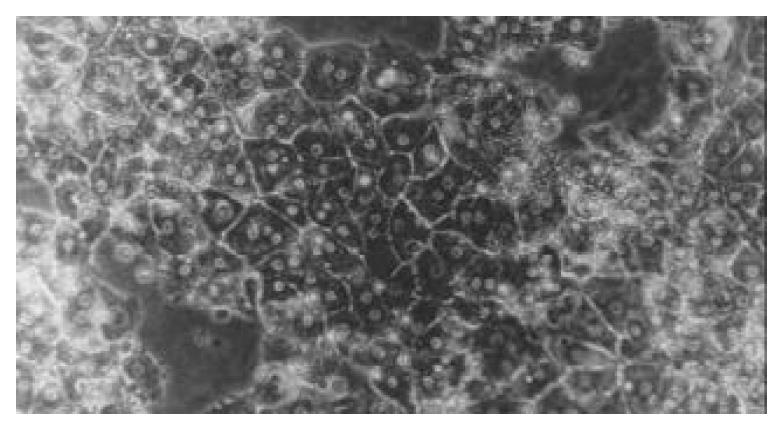
Twenty-four hours after overlaying the upper-layer of the gel, the cells still remained polygonal in shape. Over the culture period, polygonal characteristics were much more typical, and showed unique nuclei, most of which were binuclei. Another unique appearance was that the cellular border was clear and bright among the cells, indicating formation of bile canaliculi. The reconstructed hepatocytes had a cellular polarity and a plate-like structure, and maintained their characteristics till the end of culture (Figure 4).
Figure 4 Morphology of piglet hepatocytes cultured in sand-wich configurations on d 5 (phase-contrast microscope ×200).
Urea synthesis by rat hepatocytes cultured in collagen gel mixture
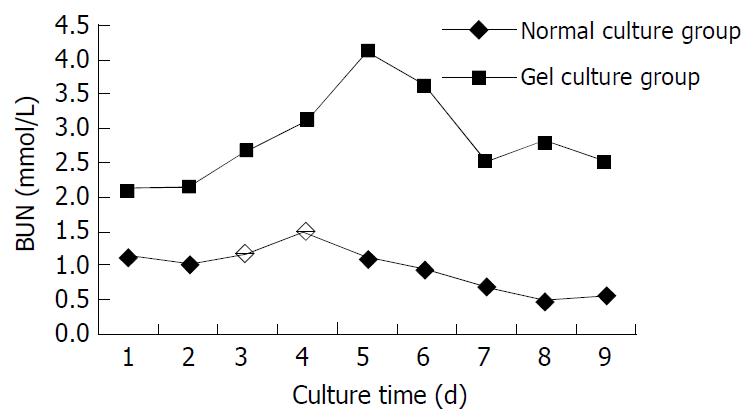
Figure 5 shows the BUN level in supernatant of the medium by dynamic measurement. The BUN level in collagen gel mixture culture group was higher than that in the normal culture group throughout the period, and was significantly higher on d 3, 4, 5,7 and 8 (n = 5, P < 0.05).
Figure 5 BUN level in supernatant of medium.
Albumin secretion of piglet hepatocytes
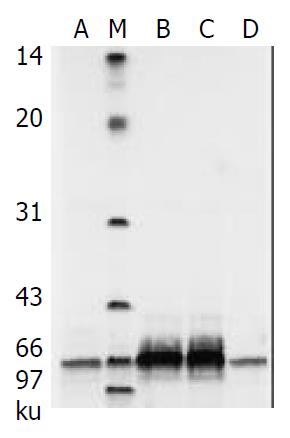
Porcine albumin released from piglet hepatocytes was detected by SDS-PAGE during the procedure of sandwich culture and high rates of urea biosynthesis were maintained throughout this period (Figure 6).
Figure 6 Albumin secretion in cultured piglet hepatocytes by SDS-PAGE analysis.
M: Low-range protein molecular weight markers; lanes A, B, C, D are culture media on days 1, 3, 5, 7.
LDH-release
The amount of LDH released from hephatocytes cultured in collagen gel was detected to be an average of 106 ± 17.4 U/L by dynamic measurement in supernatant of the medium. The amount of LDH in supernatant of the medium with sandwich culture fluctuated between 24.8-58.6 U/L, and was not significantly different (P > 0.05) at various batches. LDH release was declined both in collagen gel mixture and in sandwich culture and remained at a similar low level towards the end of culture.
DISCUSSION
Along with the improvement in the techniques of enzyme-digested hepatocyte isolation in recent years, hepatocyte culture has been applied extensively. However, since the complexity of liver structure and function as well as the existence of specificity of hepatocytes in vivo, isolating hepatocytes in vitro reguires very strict conditions. Monolayer attachment culture model is not suitable for hepatocytes in vivo, it only meets the general experimental requirements so far. In order to overcome this difficulty, enormous researches have been carried out to improve the media, supplements, growth factors, matrix or culture methods in hepatocyte culture. A series of methods have been developed, such as specified medium, addition of traceelements, transferrin, insulin, HGF, EGF, coated specific matrix, and the use of microcarrier as well as mixed culture[21-28]. However, a mature technique meeting the specific requirements of research in bioartificial liver supporting system requires a large scale of high density, high activity, and long-term hepatocyte culture. This has not been developed so far. Therefore, the exploration of hepatocyte culture with high activity becomes an important subject in this research field.
It is known that normal hepatocytes appear as a three dimensional structure arranged in the liver lobules. There exists a complicated connection between hepatocytes, parenchymal-nonparenchymal cells, and hepatocytes-extracellular matrix. Among them, the matrix has been found to play an important role in maintaining hepatocytic functions. A large number of researches have shown that using collagen to fix hepatocytes in the manner of sandwich configuration could create a matrix environment close to that in vivo, and availed thus, hepatocytes of growth and their specific function. In this study, freshly isolated hepatocytes from newborn Chinese experimental piglets were cultured between double layers of collagen sandwich configuration, and the hepatocytes were found to have good functions of protein secretion and maintained their cytomorphological characteristics with little LDH-leakage. This illustrated that double layers of collagen sandwich configuration could provide a fairly good cultural and growth condition to piglet hepatocytes with high activity. Even though the double layers of collagen sandwich configuration technique could be used in highly active hepatocyte culture, it is still a monolayer attachment culture, the liver plate so formed is still unlike the normal three dimensional structure of hepatocytes in vivo, and also lacks connections with other kinds of cells in the liver. In addition, the cell density is not high, and operation is not convenient, requiring digestion twice. Thereby, the double layers of collagen sandwich technique might not be suitable for the study of bioartificial liver supporting system and hepatocyte transplantation.
Collagen gel mixture is a novel gel immobilizing technique. In this method, the hepatocytes, collagen and medium are mixed and gelled after seeding. The medium was added on its top. Our study showed that rat hepatocytes cultured by such a method could uniformly mix in collagen gel, showing high-density and a three dimensional structural characteristic. During the culture period, the urea synthesizing ability of rat hepatocytes cultured by collagen gel mixture was obviously superior to that of hepatocytes cultured ordinarily and the LDH-release reflecting the hepatocyte activity was close to that of hepatocytes cultured ordinarily. This illustrated that collagen gel mixture could provide fine hepatocytes culture and growth with high density and high activity. The greatest advantage of this culture method is that the structure of cultured hepatocytes is closer to the normal three dimensional hepatocyte structure in vivo with the characteristics of high density and more extensive connections between hepatocytes and other cells. Therefore, collagen gel mixture immobilization may become one of the effective methods for hepatocytes culture in the bioartificial liver supporting system.