Published online Mar 1, 2004. doi: 10.3748/wjg.v10.i5.630
Revised: September 28, 2003
Accepted: October 7, 2003
Published online: March 1, 2004
AIM: To investigate the immunotherapeutic potential of vaccine consisting of dendritic cells (DCs) pulsed with total RNA from MFC gastric cancer cells.
METHODS: DCs were prepared from the spleens of strain 615 mice by magnetic cell sorting (MACS). After culture for 24 h, DCs were pulsed with total RNA from MFC gastric cancer cells. Mice of one group were immunized with tumor RNA pulsed DC (RNA/DC) at the dosage of 1 × 106 on d 14 and 7 by s c inoculation before tumor implantation. Mice of another group were immunized with unpulsed DC (UDC) at the same dosage on days as the RNA/DC group. The third group of control mice was untreated. On d 0, all the mice were challenged with s c injections of 5 × 105 MFC gastric cancer cells. After inoculation, the mice were monitored closely with respect to tumor growth. Activities of NK cells in PBL and splenocytes and CTL were tested.
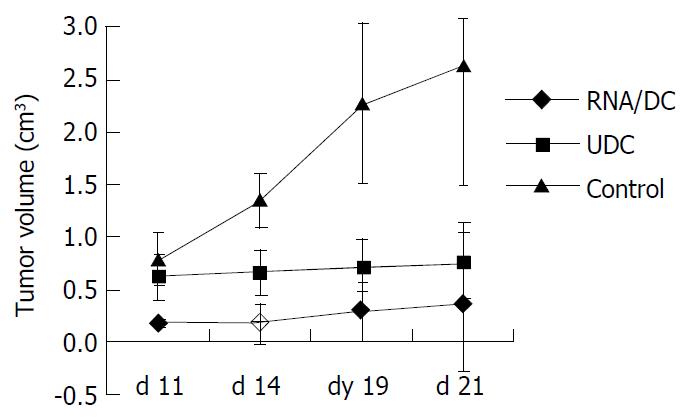
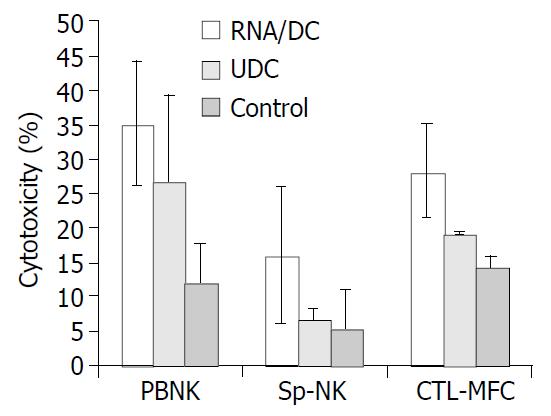
RESULTS: On d 21 after tumor cell inoculation, the mice of control group manifested the largest tumors with volume at a mean of 2.6323 ± 1.1435 cm3, followed by the UDC and RNA/DC groups with mean volumes at 0.7536 ± 0.3659 cm3 and 0.3688 ± 0.6571 cm3, respectively. The activities of NK cells in PBL and splenocytes in RNA/DC group were 66.2% and 65.4%, respectively, higher than that in the control group. The tumor specific CTL activity in RNA/DC group was 49.5%, higher than that in the control group.
CONCLUSION: The tumor vaccine with DCs pulsed with total RNA from gastric cancer cells possesses the ability to stimulate tumor specific CTL activity and to establish anti-tumor immunity when administered in vivo.
- Citation: Liu BY, Chen XH, Gu QL, Li JF, Yin HR, Zhu ZG, Lin YZ. Antitumor effects of vaccine consisting of dendritic cells pulsed with tumor RNA from gastric cancer. World J Gastroenterol 2004; 10(5): 630-633
- URL: https://www.wjgnet.com/1007-9327/full/v10/i5/630.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i5.630
T cells, in particular CD8+ cytotoxic T lymphocytes (CTLs), are regarded as the principal effectors of anti-tumor immunity. Several studies have demonstrated that tumor-specific CTL can be induced to recognize peptide epitopes presented on the tumor cell surface in the context of MHC class I molecules. Tumors, however, have evolved various mechanisms to escape from host immune surveillance, such as loss of class I or antigen variants, secretion of immuno-suppressive agents, or development of antigen-specific T cell clonally anergy due to lack of co-stimulation[1].
The dendritic cell (DC) system of antigen-presenting cells (APCs), is the initiator and modulator of immune response[2]. DCs are efficient stimulators of B and T lymphocytes. DCs have been known to be highly specialized antigen-presenting cells and principal activators of resting or naive T cells in vitro and in vivo, capable of efficiently transporting antigens[3].
Immature DCs are capable of capturing and processing antigens extensively, they are also able to internalize apoptotic cells by binding to them via adhesion molecules[4]. After the uptake and processing of antigens, DCs leave peripheral tissues and migrate to lymphoid organs in order to present antigens to T lymphocytes. In lymph nodes, DCs form clusters with T lymphocytes by means of various adhesion molecules, in particular CD54 (ICAM-1), CD102 (ICAM-3) and CD58 (LFA-3), that are highly expressed by activated DCs[5]. The DC/T cell interaction is stabilized by the specific ligation of T cell receptors (TCR) of the MHC-peptide complex, which delivers the first activation signal to T lymphocytes. The second costimulatory signal is absolutely necessary to allow T cell activation and proliferation. This signal is mediated by the interaction of CD80 (B7-1) and CD86 (B7-2), both of which present on DC, with T cell CD28. Expression of a large amount of MHC costimulatory molecules and production of IL-12 by mature DCs turn them into very potent professional APCs[6]. DCs pulsed with tumor antigen can induce specific CTL activity[7].
The aim of the present study was to investigate the ability of DC pulsed with tumor RNA to stimulate specific CTL reaction and the potential usefulness of DC-based vaccines for the treatment of cancer patients.
Seven to eight wk-old strain 615 mice (H-2Kk) used in these experiments, were obtained from the Chinese Academy of Medical Sciences (Beijing, China). The murine gastric carcinoma cell line MFC derived from strain 615 mouse was provided by the Chinese Academy of Medical Sciences. MFC was cultured in MPRI-1640 medium containing 100 g/L FCS.
Spleen cells were prepared and treated with collagenase D. Splenocytes were labeled with mouse CD11C+ DC MicroBeads (MACS, Miltenyi Biotec) according to the manufacturer’s manual. In brief, 108 splenocytes were resuspended in 400 µL buffer and 100 µL of MACS CD11c MicroBeads was added. After thoroughly mixed, the splenocytes were incubated for15 min at 6-12 °C, followed by a wash with 10-20× labeling volume of buffer. Cells were resuspended in 500 µL of buffer and applied onto the MS+/MS+ column to allow removal of the negative cells. Three rinses with 500 µL of buffer were performed prior to removal of the column from the separator. The column was placed in a collection tube, and 1ml of buffer was flushed into the column to secure the positive cells. The positive cells were cultured in IMDM containing 200 mL/L FCS, 100 ng/mL IL-4, 100 ng/mL GM-CSF, 10 ng/mL TNF-α for 24 h, then examined by scanning electron microscope and flow cytometry.
Tumor total RNA was isolated from actively growing tissue culture cells, 1 × 107 MFC murine gastric cancer cells were lysed in 1 mL of Trizol reagent (Life Technologies) prepared according to the protocol provided by the manufacturer. One mL of Trizol reagent was added to 1 × 107 of MFC cells which were well mixed well prior to incubation for 5 min at room temperature. Then 0.2 mL of chloroform was added and the mixture was thoroughly mixed and incubated for 3 min at room temperature. After centrifugation of 12000 g for 15 min at 4 °C, contents were transferred in the aqueous phase to a fresh tube, then 500 µL of isopropyl alcohol was added and mixed well, followed by incubation at room temperature for 10 min and centrifuged at 12000 g for 10 min at 4 °C. The RNA pellet was washed with 1 mL of 750 mL/L ethanol, and redissolved in RNase free water.
The procedure used for pulsing DCs with tumor RNA was described by Boszkowsk[8]. In brief, DCs were washed twice in Opti-MEM medium (GIBCO BRL). Cells were resuspended in Opti-MEM medium at 2-5 × 106 cells/mL and transferred into 15 mL polypropylene tubes (Falcon). The cationic lipid, DOTAP (Boehringer Manheim), was used to deliver RNA into the cells, and 25 µg of total RNA in 500 µL Opti-MEM and 50 µg of DOTAP in 500 µL Opti-MEM were mixed in 12 × 75 mm polystyrene tubes at room temperature for 20 min. The complex was added to the DCs and incubated at 37 °C for 2-4 h.
Tumor RNA pulsed DCs (RNA/DC) and unpulsed DCs (UDC) were collected, washed twice with PBS and then resuspended in PBS. Mice from the respective treatment groups were immunized with these preparations. Each mouse received the designated dose of 1 × 106 DCs s c with an intervening period of 7 d before administration of the second dose. The mice untreated were used as control. Twelve mice were in RNA/DC group, 10 mice in UDC group, and 12 mice in control group.
Seven days after vaccination with DCs for the second and last time, each mouse in the RNA/DC group received inoculation of 5 × 105 MFC stomach cancer cells subcutaneously. The animals were closely monitored until the first palpable tumor appeared. Thereafter, two-dimensional tumor measurements were made using calipers, the measurements were recorded every 3-4 d.
Three weeks after tumor challenge, all the mice were sacrificed. Their spleens were removed and placed in PBS. The splenocytes were depleted of red blood cells through incubation in 8.4 g/L ammonium chloride for 10 min at 37 °C. The samples were then washed twice with PBS. The cells obtained were resuspended with RPMI-1640 containing 100 g/L FBC, 2β- mercaptoethanol and 10 u/mL IL-2. Splenocytes were stimulated with irradiated MFC gastric cancer cells (5000 rads) in the ratio of 10:1 (E:T). The samples were then cultured for 5 days and CTLs from each culture were tested. Cell mediated lysis was confirmed in vitro using standard chromium (51Cr)-release assay.
When the mice were sacrificed, p40 subunits of IL-12 in serum were measured using standard ELISA (R & D).
DCs were cultured in IMDM containing 200 g/L FCS, 100 ng/mL IL-4, 100 ng/mL GM-CSF, 10 ng/mL TNF-α for 24 h after sorted by mouse CD11C+ DC MicroBeads. DCs phenotypes were detected by flow cytometry with H-2KK, I-EK, CD80, and CD 86 (Table 1).
| Before culture (%) | After culture (%) | |
| H-2KK | 92.1 | 91.6 |
| I-EK | 28.1 | 59.2 |
| CD80 | 21.0 | 76.4 |
| CD86 | 23.1 | 61.3 |
To determine whether RNA pulsed DC could induce host protective immunity against MFC gastric cancer, strain 615 mice were immunized subcutaneously with RNA pulsed DCs or unpulsed DCs. Seven days after immunization, the mice were inoculated subcutaneously with 5 × 105 MFC murine gastric cancer cells. The results (Figure 1) showed that the mice in the control group (control mice) were all tumor positive on d 7, while the mice immunized with RNA pulsed DCs demonstrated a significant delay in tumor development (7 vs 11 d). RNA pulsed DC immunization reduced tumor incidence significantly. In the RNA/DC group, 41.7% of the mice (5/12) were free from tumor; in the UDC group, 80% of the mice (8/10) developed tumors, and all the mice (12/12)developed tumor in control group on 21 d after tumor cell inoculation. On d 21 after tumor cell inoculation, the tumor volume of the control group attained a mean of 2.6323 ± 1.1435 cm3, followed by the UDC and RNA/DC groups with mean volumes of 0.7536 ± 0.3659 cm3 and 0.3688 ± 0.6571 cm3, respectively (Figure 1).
Four weeks after immunization, splenocytes were restimulated in vitro with irradiated MFC murine gastric cancer cells for cytotoxic T lymphocyte (CTL) propagation. The results demonstrated that tumor RNA pulsed DCs induced strong tumor specific CTL production. Immunization with MFC tumor RNA pulsed DCs induced stronger lysis of MFC (Figure 2). NK cell activity in PBL or in splenocytes was examined after the mice were sacrificed, revealing that the greatest NK cell activity was seen in the RNA/DC group.
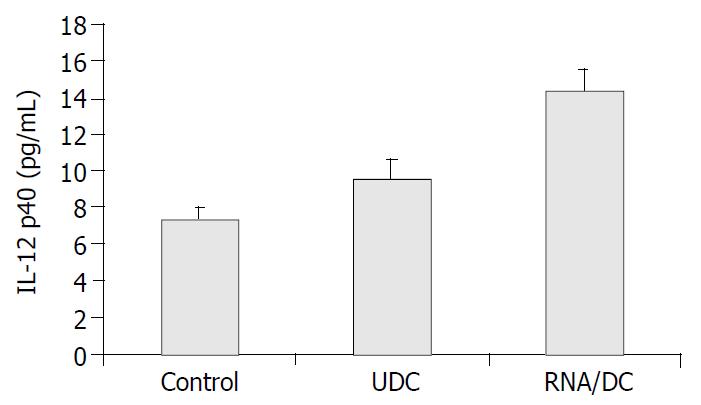
Three weeks after tumor challenge, IL-12 in serum was detected by ELISA. The results showed that the serum IL-12 level was the highest in the RNA/DC group among the three groups (Figure 3).
In recent years, DCs have been found to play an important role in the rejection of tumors by the immune system. Their infiltration of tumors has been associated with an improved prognosis for many neoplasms[9,10].
A number of tumor antigens recognized by CD8+ CTL have been identified[11]. These defined antigens could simply be added as 9-11 mer HLA-I-restricted peptides to mature DCs[12]. So, the use of peptide-pulsed DC can obtain clearly effective results, but it requires prior knowledge of patients’ HLA types and the sequence of the relevant peptide epitopes. It has been suggested that the use of longer peptides or whole proteins, which are not directly present but are endocytosed and processed, is a better way to generate a fully competent DC vaccine since it allows selection by the APC of the optimal 9-mer peptide for presentation[13]. After cloning, these tumor antigens can also be used as cDNA in appropriate vectors to load DCs. But there are three major restrictions when defined tumor antigen is used. First, effective TSA or TAA is not identified in a large number of cancers, especially in gastric cancer. Second, it is clear that immunotherapy approaches directing against a unique antigen favor the selection of a tumor mutant that has selectively lost its ability to present efficiently to the defined antigen[14]. Third, the acquisition of antigen-specific CTL in vitro is not a guarantee of the development of a curative immune response in vivo.
To bypass these disadvantages, several alternative methodologies have been developed. Unfractionated MHC-I-presented peptides could be eluted from tumor cells and loaded onto DC [1]. DCs could also be pulsed with whole tumor antigens, such as cell lysates[15], cell extracts[16], apoptotic cells[4,17], total RNA or mRNA[7]. The method of fusing tumor cells with DCs has also been explored[18,19]. The recent demonstration of cell-contact generated by short-term coculture of DC with tumor cells without cell fusion could result in a potent immunogen and was particularly relevant[20]. DCs pulsed with tumor RNA may thus be a potential effective means to induce host T cell-mediated anti-tumor responses. Boczkowski et al[8] have recently demonstrated that vaccination with tumor RNA pulsed DCs could elicit T cell protective immunity against tumor challenge and induce immune rejection of established tumors.
Gastric cancer is one of the tumors with very high heterogeneity. Host immune status in patients with gastric cancer is usually poor. Up to the present, immunotherapy for gastric cancer has not been clearly effective. The use of DC-based tumor vaccine can provide a glimmer of hope for patients with gastric cancer. In this experiment, we used tumor total RNA as tumor whole antigen to pulse DCs. All the antigens were processed and selectively presented to T cells by DCs. The results following the use of tumor total RNA pulsed DCs as vaccine, indeed, induced anti-tumor immune responses by stimulating NK cells and tumor-specific CTL activity in the mouse.
For clinical trials of DC-based vaccine for cancer, other matters must be considered. The first is the source of DCs for clinical use. The second is the choice of tumor antigens and method for antigen loading of DCs. Third, the DC dose-response relationship should be determined using individual cell preparation methods. Finally, the optimal route and frequency of administration need to be determined.
We want to thank Dr. Dong-Qing Zhang, Shanghai Institute of Immunology for his good advice, and Yi Zhang, research assistant, for her work and Dr. Ming-Jun Zhang for his help in animal experimentation.
Edited by Wang XL
| 1. | Zitvogel L, Mayordomo JI, Tjandrawan T, DeLeo AB, Clarke MR, Lotze MT, Storkus WJ. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med. 1996;183:87-97. [PubMed] |
| 2. | Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245-252. [PubMed] |
| 3. | Constant S, Sant'Angelo D, Pasqualini T, Taylor T, Levin D, Flavell R, Bottomly K. Peptide and protein antigens require distinct antigen-presenting cell subsets for the priming of CD4+ T cells. J Immunol. 1995;154:4915-4923. [PubMed] |
| 4. | Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359-1368. [PubMed] |
| 5. | Scheeren RA, Koopman G, Van der Baan S, Meijer CJ, Pals ST. Adhesion receptors involved in clustering of blood dendritic cells and T lymphocytes. Eur J Immunol. 1991;21:1101-1105. [PubMed] |
| 6. | Tarte K, Klein B. Dendritic cell-based vaccine: a promising approach for cancer immunotherapy. Leukemia. 1999;13:653-663. [PubMed] |
| 7. | Zhang JK, Li J, Chen HB, Sun JL, Qu YJ, Lu JJ. Antitumor activities of human dendritic cells derived from peripheral and cord blood. World J Gastroenterol. 2002;8:87-90. [PubMed] |
| 8. | Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465-472. [PubMed] |
| 9. | Ishigami S, Aikou T, Natsugoe S, Hokita S, Iwashige H, Tokushige M, Sonoda S. Prognostic value of HLA-DR expression and dendritic cell infiltration in gastric cancer. Oncology. 1998;55:65-69. [PubMed] |
| 10. | Saito H, Tsujitani S, Ikeguchi M, Maeta M, Kaibara N. Relationship between the expression of vascular endothelial growth factor and the density of dendritic cells in gastric adenocarcinoma tissue. Br J Cancer. 1998;78:1573-1577. [PubMed] |
| 11. | Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183:725-729. [PubMed] |
| 12. | Tsai V, Southwood S, Sidney J, Sakaguchi K, Kawakami Y, Appella E, Sette A, Celis E. Identification of subdominant CTL epitopes of the GP100 melanoma-associated tumor antigen by primary in vitro immunization with peptide-pulsed dendritic cells. J Immunol. 1997;158:1796-1802. [PubMed] |
| 13. | Nieda M, Nicol A, Kikuchi A, Kashiwase K, Taylor K, Suzuki K, Tadokoro K, Juji T. Dendritic cells stimulate the expansion of bcr-abl specific CD8+ T cells with cytotoxic activity against leukemic cells from patients with chronic myeloid leukemia. Blood. 1998;91:977-983. [PubMed] |
| 14. | Ikeda H, Lethé B, Lehmann F, van Baren N, Baurain JF, de Smet C, Chambost H, Vitale M, Moretta A, Boon T. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199-208. [PubMed] |
| 15. | Tang ZH, Qiu WH, Wu GS, Yang XP, Zou SQ, Qiu FZ. The immunotherapeutic effect of dendritic cells vaccine modified with interleukin-18 gene and tumor cell lysate on mice with pancreatic carcinoma. World J Gastroenterol. 2002;8:908-912. [PubMed] |
| 16. | Asavaroengchai W, Kotera Y, Mulé JJ. Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc Natl Acad Sci USA. 2002;99:931-936. [PubMed] |
| 17. | Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86-89. [PubMed] |
| 18. | Zhang JK, Li J, Zhang J, Chen HB, Chen SB. Antitumor immunopreventive and immunotherapeutic effect in mice induced by hybrid vaccine of dendritic cells and hepatocarcinoma in vivo. World J Gastroenterol. 2003;9:479-484. [PubMed] |
| 19. | Zhang J, Zhang JK, Zhuo SH, Chen HB. Effect of a cancer vaccine prepared by fusions of hepatocarcinoma cells with dendritic cells. World J Gastroenterol. 2001;7:690-694. [PubMed] |