INTRODUCTION
The primary management of esophageal carcinoma is surgical resection, and the stomach is the organ most often chosen for substitution following removal of esophagus for carcinoma. However, following the resection of esophageal carcinoma after distal subtotal gastrectomy, the replacement of esophagus mostly selected other organs, such as jejunum or colon[1-11]. Since we first applied this new technique to use the residual stomach in patients all with previous distal subtotal gastrectomy in 1982[12,13], we have treated 85 patients by such approach, and will report it as follows.
MATERIALS AND METHODS
Clinical data
There were 74 men and 11 women with age ranging from 34 to 78 years (mean 60.3 years). The interval between the time of subtotal gastrectomy and the time when the patients were diagnosed having carcinoma of esophagus ranged from 7 to 29 years, averaging 15 years. According to the procedures of subtotal gastrectomy, two cases were classified as Billroth I type, 83 cases Billroth II type, including 27 cases undergoing gastrectomy posterior to colon, and 58 cases anterior to colon. The digestive functions in all of the patients were approximately normal. All the patients presented with the symptoms of dysphagia to some degree with only 42.4% (36/85) being able to take semi-fluiddiet. The average body weight of the patients was 47.7 ± 10.2 kg. With regard to the classification of Performance Status, 30.6% (26/85) patients belonged to class 0-1, and 69.4% (59/85) to class 2-4.
In terms of the location of the tumor, 4 cases were localized in upper thoracic esophagus, 62 cases in mid thoracic, and 19 cases in lower thoracic. With regard to gross type, 60 patients belonged to medullary type, 15 ulcerative type, six constrictive type, three fungating type and one intraluminal type. All of cases received barium meal examination of gastrointestinal tract to measure the maximal diameter of residual stomach that was from the fundus to the gastrojejunal anastomosis, and no lesion was found in the residual stomach. The diameter ranged from 5 cm to 9.2 cm, mean 7.9 cm. Forty-three cases in this group were further examined by fiber-gastroscopy, which revealed nothing pathological in their residual stomachs.
All the patients underwent the resection of involved portion esophagus through left thoracotomy under the intratracheal anesthesia, except for 7 cases being found to have unresectable tumors, in which 5 cases had the local tumors infiltrating the aortic arch or / and bronchus, and two cases were found to be accompanied with metastasis of liver and extensive spread of intra-abdominal cavity.
Seventy-eight cases underwent the resection of the carcinoma of esophagus with a resectability rate of 91.8% (78/85), including 64 cases for radical resection and 14 cases for palliative resection. As for length of the involved segment of esophagus, 21 cases were shorter than 3 cm, 17 cases ranged from 3-5 cm, and 40 cases longer than 5 cm. The maximal diameters of residual stomachs measured from the fundus to the gastrojejunal anastomosis, ranged from 6 cm to 11.3 cm, mean 9.1 cm which was in contrast to X-ray examination. According to the location of anastomosis sites, 5 cases were localized in neck, 51 above aortic arch, and 22 below aortic arch. Sixty-five patients were anastomosed by handwork, 13 by mechanical anastomat, including 10 above aortic arch and 3 below aortic arch. During the operation, a silica-gel catheter with a metal guiding core was inserted into the jejunum through nostril for 30 cases[14]. Those patients were fed with mixed milk or nutritional fluid through the nasal feeding catheter one day after operation. The patients had a meal 5-10 d following operation (mean 7 d). The span of hospitalization ranged from 14 to 52 d (mean 14.6 d).
Procedure of operation
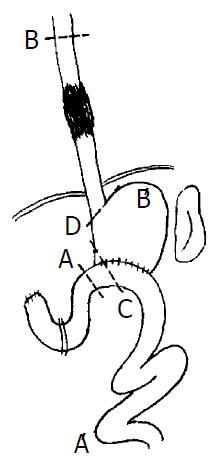
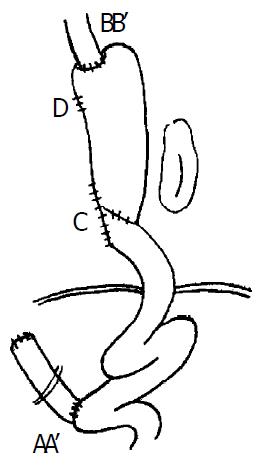
Our approach was through a left thoracotomy incision. The tumor was examined and if it appeared to be resectable, the left diaphragm was opened and the exploration of abdominal cavity was done. After examining the operative condition of previous subtotal gastrectomy, the spleen, splenic hilus and pancreatic tail were dissociated from the back of peritoneum. Meanwhile, two to four short gastric blood vessels should be preserved to provide adequate blood supply for the residual stomach. The residual stomach, afferent and efferent loops of jejunum were mobilized from peripheral cohesion, and the previous gastrojejunal anastomosis was brought into the left thorax, the afferent loop of jejunum close to gastrojejunal anastomosis was transected and the gastric end was closed. If the part of gastrojejunal anastomosis was removed at the same time, the length of the residual stomach may be prolonged (Figure 1: C). The afferent loop is rejoined to the efferent loop 30 cm below the original gastrojejunostomy (Roux-en-Y method, Figure 2: AA’). The cardia was transected and closed (if mechanical approach was used, the cardia was not closed temporarily, Figure 1: D). The residual stomach, gastrojejunal anastomosis, efferent of jejunum, spleen, tail of pancreas were all transposed into the left thoracic cavity. The esophagus was mobilized and the upper end of esophagus resected 5 cm above the tumor as described in Sweet’s esophago-gastrostomy (Figure 2: BB’). If the staple was used, the esophagus was anastomosed to the fundus of the substitution through the cardiac, and the end of cardiac was shut soon after. The splenic ligament was fixed firmly to the thoracic wall to reduce the tension on the esophagogastric anastomosis. If the anastomosis was performed in neck, the fundus of the residual stomach could be brought up into the neck through thoracic cavity or the bed of esophagus. The approach of the operation is represented diagrammatically in Figure 1 and Figure 2. Preoperative and postoperative barium meal examinations, postoperative roentgenogram of chest and field of operation in one of the patients are shown in Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7.
Figure 1 Resection range.
Figure 2 Postoperative situation.
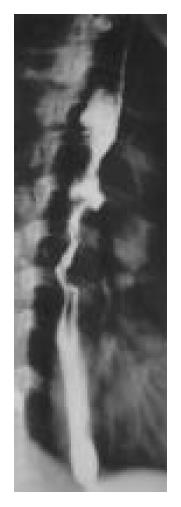
Figure 3 Preoperative barium.
Figure 4 Preoperative barium meal examination, showing meal examination, showing re-mid thoracic esophageal tumor.
sidual stomach.
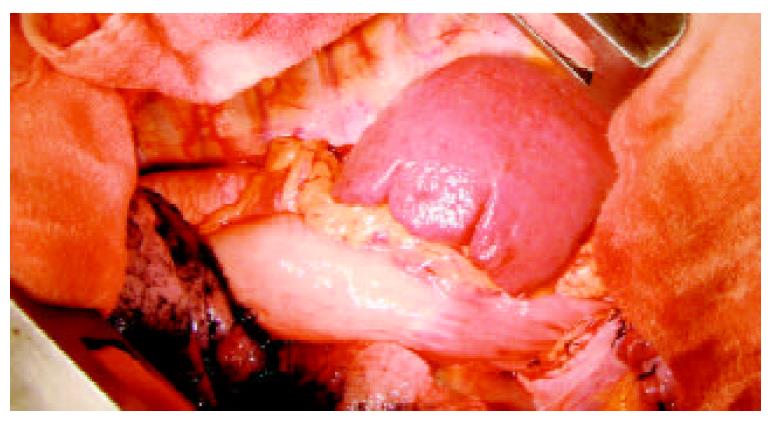
Figure 5 Field of operation, showing the spleen in the left tho-racic cavity.
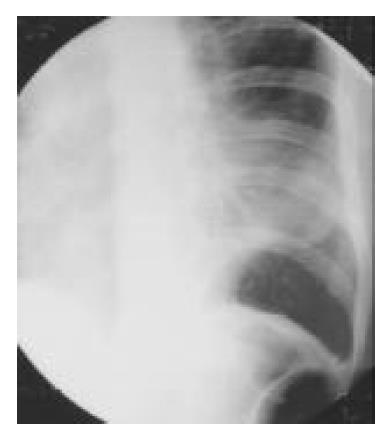
Figure 6 Postoperative roentgenogram of chest, showing the spleen shadow in the left thoracic cavity.
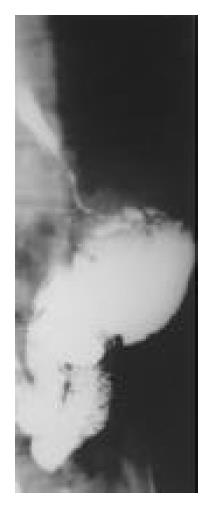
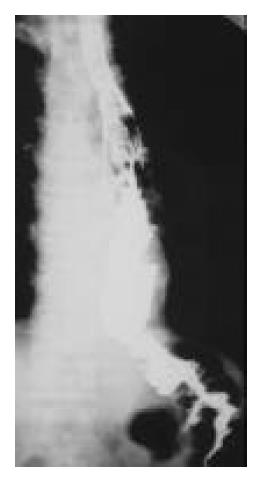
Figure 7 Postoperative barium meal examination, showing the esophagogastric anastomosis above the aortic arch, the residual stomach with previous gastrointestinal anastomosis, and the Roux-en-Y anastomosis.
Follow-up and statistical analysis
All the patients were followed up after operation. The patients failed to be followedup were deemed as death according to the last time they could be contacted. The survival rate was calculated on the basis of Kaplan-Meier procedure by statistical software SPSS 10.0. The condition of eating, the body weight and the Performance Status for the patients recruited in our study 3 months after the operation. Enumeration data and measurement data were analyzed by χ2 and t test, respectively.
RESULTS
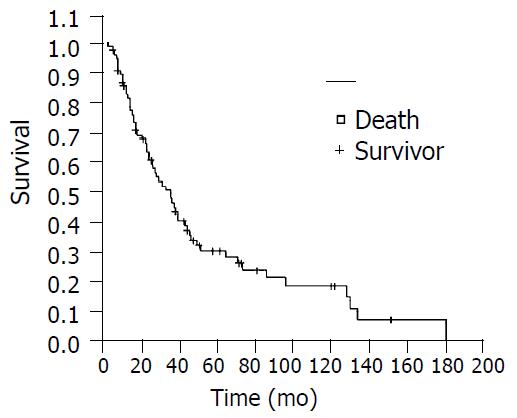
Seventy-eight patients received resection of the tumor, including 24 cases having grade I of squamous carcinoma, 47 cases having grade II of squamous carcinoma, 4 cases grade III of squamous carcinoma, 2 cases adenocarcinoma and 1 case carcinosarcoma. There were two cases having positive resection margins. With regard to lymph node status, 34 patients were complicated with metastasis of lymph node, and 43 patients without. According to UICC1997 PTNM stage, 5 patients were classified as stage I, 30 stage II, and 43 stage III. The complication rate following operation was 10.3% (8/78), with two cases suffering from arrhythmia, two cases having pneumonia and four cases having anastomostic leakage. All these patients with complications were cured through conservative management without operation death. The follow-up rate was 91.0% (71/78), six patients lost contact. The 1, 3, 5, and 10yr survival rate were 85.7%, 50.7%, 30.6% and 18.8%, respectively (Figure 8). The patients undergoing resection could take semi-fluid diet, accounting for 92.3% (72/78). Among all the patients, 84.7% (72/85) could have 300 ± 500 mL volume of semi-fluid diet. The mean body mass was 49.8 ± 8.9 kg. Compared with the previous body mass, 41.0% (32/78) patients got a body weight gain after operation. In term of the grade of Performance Status, 72.9% (62/85) patients had grade 0-1, and 27.1% (23/85) grades 2-4. Apart from the body mass, the difference between the index of quality of life before operation and the comparative index after operation was statistically significant (P < 0.05).
Figure 8 Kaplan-Meier survival curve.
DISCUSSION
Cancer of esophagus in Chaoshan region is prevalent with a high incidence, so are the gastroduodenal diseases, especially the peptic ulcer. Therefore, there are some patients with esophageal carcinoma after subtotal gastrectomy in this area. The incidence rate of these patients was 2.3% (85/3632) compared with the patients with esophageal carcinoma treated surgically in our hospital in the same period, which demonstrates the management of these patients should also be emphasized. The average interval between gastrectomy and diagnosis of esophageal carcinoma was 15 years. The mean age of the patients was 60.3 years, with no significant difference compared with other patients suffering from esophageal carcinoma without subtotal gastrectomy. So it may be suggested the development of carcinoma of esophagus is not obviously correlated with the history of surgical operation of stomach. The relationship between esophageal carcinoma and previous gastrectomy for benign ulcer disease are still not clear[9,10,15-19].
As for the influence of previous operation, the colon or jejunum is the most popular choice as a substitution in such patients. This approach is complicated and can greatly affect the postoperative quality of life[13,20,21]. For this reason, some patients were not accessible to resection, but were treated by radiotherapy. When the demand of the resection of esophageal carcinoma was met, the procedure of using the residual stomach to reconstruct the digestive tract, is the best choice, which not only facilitates the recovery of digestive function following operation, but also improves the quality of life in patients with esophageal carcinoma after previous subtotal gastrectomy. In the process of this procedure, the residual stomach must have sufficient length for lifting, and good blood supply. The blood supply of the residual stomach originated from the short artery. Transferring spleen, tail of pancreas to thoracic cavity can provide the residual stomach with good blood supply, and benefit the lifting length of it. Amputating at the jejunual afferent loop near anastomosis, and making the jejunual Roux-Y anastomosis, fully dissociating the previous operative cohesion could also prolong the lifting length and decrease the lifting tension of the residual stomach. After being fully dissociated, the residual stomach can be lifted up by about 20 cm. Four cases were complicated with anastomotic leakage in the group, including 3 cases with thoracic leakage[13] occurring during the early application of this technique. The causes of the thoracic leakage might be related to the local infection and suturing skill. The cause of the other case with anantomotic leakage in neck probably was connected with the tension of the residual stomach. Recently, we replaced manual manipulation with mechanical anastomosis, using staple to close the cardiac and the lesser curvature of stomach. This is better for the extension of the residual stomach, reducing the traction and tension of anastomosis. At the early period after operation, the patients were offered nasal feeding nutrition, which was conducive to the rehabilitation and reduction of complications for patients after resection.
So far, there has been no clear report on the benefit of this procedure, which preserves the residual stomach and anastomoes it to esophagus, in terms of the long-term effect and quality of life for the patients with esophageal carcinoma after subtotal gastrectomy[21-29]. Compared with the management of the other patients with esophageal carcinoma in corresponding period, the difference between the means of operation and the findings of pathology in this group was not significant. The 5- and 10-year survival rates were 30.6% and 18.8% respectively. In comparison of the long-term survival rate of our group and those of the other big groups[10,30-33], no significant difference could be found. All these demonstrate that such an approach can achieve a good efficacy. The patients were satisfied with their quality of life, and the majority of them could have semi-fluid diet. Over 50% of them had a body mass gain. The grade of Performance Status of them also improved greatly. In conclusion, the above evidences further confirm that in the management of the patients with carcinoma of esophagus after previous subtotal gastrectomy, the technique, which used the residual stomach to reconstruct the alimentary tract, not only accomplished a rather good long-term survival rate, but also improved the quality of patients’ life. So it is a simple operative approach with few trauma and good results.