Published online Dec 15, 2004. doi: 10.3748/wjg.v10.i24.3650
Revised: April 9, 2004
Accepted: April 16, 2004
Published online: December 15, 2004
AIM: To estimate the amount of apoptosis among healthy HBsAg carriers, patients with chronic HBV infection treated with lamivudine and patients with chronic HCV infection treated with interferon alpha and ribavirin. Activity of apoptosis was evaluated by serum sFas/sFasL concentration measurement. Moreover dependence between apoptosis and HBV-DNA or HCV-RNA levels was studied.
METHODS: Eighty-six persons were included into study: 34 healthy HBsAg carriers, 33 patients with chronic HBV infection and 19 patients with chronic HCV infection. Serum levels of sFas/sFasL were measured by ELISA assay. HBV-DNA and HCV-RNA were measured by RT-PCR assay. Levels of sFas/sFasL were determined before and 2 and 12 wk after therapy in patients with chronic hepatitis B and C infection. HBV-DNA or HCV-RNA was detected before treatment and 6 mo after treatment.
RESULTS: Twenty-four (71%) healthy HBsAg carriers showed HBV-DNA over 105/mL, which was comparable to the patients with chronic hepatitis B. Independently from HBV-DNA levels, the concentration of sFas among healthy HBsAg carriers was comparable to healthy persons. Among patients with chronic hepatitis B and C, the concentration of sFas was significantly higher in comparison to healthy HBsAg carriers and healthy persons. In chronic hepatitis B patients the concentration of sFas was decreased during lamivudine treatment. Among chronic hepatitis C patients the concentration of sFas was increased during IFN alpha and ribavirin treatment. sFasL was not detected in control group. Furthermore sFasL occurred more frequently in chronic hepatitis C patients in comparison to chronic hepatitis B patients.
CONCLUSION: There are no correlations between apoptosis and HBV-DNA levels. However ther is an association between apoptosis and activity of inflammation in patients with chronic HBV infection. Apoptosis can be increased in patients with chronic hepatitis C by effective treatment which may be a result of apoptosis stimulation by IFN-α.
- Citation: Lapinski TW, Kowalczuk O, Prokopowicz D, Chyczewski L. Serum concentration of sFas and sFasL in healthy HBsAg carriers, chronic viral hepatitis B and C patients. World J Gastroenterol 2004; 10(24): 3650-3653
- URL: https://www.wjgnet.com/1007-9327/full/v10/i24/3650.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i24.3650
Apoptosis is one of the factors prolonging inflammation in chronic hepatitis B and C. Impaired apoptosis activity or its hyperactivity may induce unfavourable course of HBV and HCV infection. Fas and FasL are proteins that regulate activity of apoptosis[1]. Fas is dominantly localized on liver cells. Activation of Fas is an after-effect of fusion with FasL lymphocyte receptors. These particles are recognised defining coefficient state of stimulation apoptosis[2,3]. The concentration of Fas/FasL in serum patients with chronic hepatitis B and C can define the success of therapy.
The aim of this study was to define the activity of apoptosis among healthy HBsAg carriers and patients with chronic hepatitis B and C. Apoptosis was defined by use of sFas and sFasL serum concentration measurements. Moreover the apoptosis activity was compared with HBV-DNA or HCV-RNA serum levels. sFas and sFasL levels were monitored during antiviral therapy and afterwards depending on treatment effectiveness.
The study included 86 persons divided into three groups: HBsAg carriers (n = 34, 15 women and 19 men, aged 20-43 years), chronic hepatitis B patients (n = 33, 10 women and 23 men, aged 18-76 years), and chronic hepatitis C patients (n = 19, 6 women and 13 men, aged 18-76 years).Criteria for inclusion of HBsAg carriers group were: HBsAg presence for at least 1 year; ALT, AST activity, concentration of bilirubin, albumin and PT in normal range in one-year period of observation (triple investigation); no changes in USG investigation of liver; absence of drug or alcohol abuse, autoimmune disease, HIV, delta virus or hepatitis C coinfection, and neoplasmatic and other serious diseases which might alter apoptosis activity. Criteria for inclusion of chronic hepatitis B patients were: HBsAg, HBeAg and HBV-DNA in serum for 6 mo; ALT activity over 80 IU; inflammatory changes in histological evaluation of liver tissue. All chronic hepatitis B patients were treated with nucleic analogue - lamivudine (Zeffix® , GlaxoSmithKline, Great Britain) in a dose of 100 mg/24 h for 12 mo.
Criteria for inclusion of chronic hepatitis C patients were: presence of anti-HCV and HCV-RNA in serum for 6 mo, ALT activity over 80 IU and inflammatory changes in histological evaluation of liver tissue.
Patients with chronic HCV infection were treated with Rebetron® (IFN-α 2 b and ribavirin, Schering-Plought, USA): IFN-α 2 b in 9 M IU/wk and ribavirin 1000-1200 mg/24 h for 12 mo.
The standard concentration of sFas and sFasL in serum was evaluated in 12 healthy persons, 8 women and 4 men, aged 26-46 years. Individuals in control group were HBs and anti-HCV negative and ALT activity in serum was within normal range during 6 mo observation.
HBsAg and HBeAg in serum were detected by MEIA test (ABBOTT, USA).
HBV-DNA was extracted from 200 μL of patients’ sera the gene elute mammalian genomic DNA Miniprep kit (Sigma, USA) for DNA isolation. Part of HVB-DNA was amplified by PCR system with pair of complement primer to conservative part of genome (sense 5’-AGGGGAGGAGATTAGGTTAA-3’ antisense 5’-AGGAGTGCGAATCCACACTC-3’) in 20 μL reaction mixture containing 200 μmol/L dNTPs, 0.4 μmol/L all primers, 1.5 mmol/L MgCl2, 1.0 U Taq polymerase (Sigma) and 4 μL DNA solution. Forty cycles of amplification were performed (at 96 °C for 30 s, at 57 °C for 60 s and at 72 °C for 60 s). The products of amplification were appointed in 20 g/L agar gel by electrophoresis, and stained by ethidium bromide. Electrophore was visualized in record system and computer analysis UVI-KS400i/Image PC (Syngen Biotech, USA). Part of solution from DNA was kept at temperature -20 °C for further stages of work.
In order to detect HBV-DNA sequences in sera of the patients, the conventional PCR method with primers to conserved pre-S/S region of the genome was used. Thermal cycling was performed using the following conditions: initial incubation at 96 °C for 120 s, and then 40 cycles at 94 °C for 30 s, at 50 °C for 30 s and at 72 °C for 60 s.
HBV-DNA concentration in sera was evaluated by real-time detection PCR based on TaqMan chemistry. Amplification was performed in 25 mL reaction mixture containing 2 × TaqMan Universal Master Mix (Applied Biosystems, USA) with uracil N’-glycosylase, 30 pmoL of forward primer, 30 pmoL of reverse primer, 30 pmoL TaqMan probe (5’-FAM) and 5 μL of isolated DNA. After incubation for 2 min at 50 °C, which enabled uracil N’-glycosylase to inactivate possible contaminating amplicons, incubation for 10 min at 95 °C allowed AmpliTaq Gold polymerase to activate and inactivate the uracil N’-glycosylase. Next cycles of PCR were moved. Number of copies was counted by interpolation from definite standard curve.
Presence of anti-HCV antibodies in serum was detected by MEIA (ABBOTT, USA). Test consisted of recombined core proteins for HCr43, structural proteins for c 200 (NS3 and NS4) and unstructured proteins for c 100-3 (NS4) and NS5.
HCV-RNA was isolated from 50 μL blood of patients. Parts of viral HCV-RNA were amplified in arrangement of RT nested PCR reaction with two peers nested primers, complementary to conservative part of genome virus (external primers: sense 5’-TCTAGCCATGGCGTTAGTATGAGTGT-3’, antysense 5’-CACTCGCAAGCACCCTATCAGGCAGT-3’; internal primers: sense 5’-GGCGACACTCCACCATAGAT-3’, antysense 5’-GTGCACGGTCTACGAGACCT-3’). Stages of reverse transcription as well as first PCR were executed in one test-glass in 20 μL reaction mixture containing 200 mmol/L dNTPs, 1 mmol/L from each steam external primer, 1.5 mmol/L MgCl2, 0.75 mmol/L MnSO4, 2.0 At Tth of polymerase (EPICENTRE® , USA) as well as 8 mL RNA. After 30 min of incubation at 60 °C for 40 cycles of amplification were executed (at 94 °C for 30 s, at 55 °C for 60 s, at 72 °C for 60 s). A 1 mL product of first PCR was amplified with par of internal primers in 20 mL reaction mixture containing 200 mmol/L dNTPs, 0.4 μmol/L from each primer, 1.5 mmol/L MgCL2, 1.0 U Taq of polymerase (Sigma, USA). Afterwards, 40 cycle of amplification were performed (at 94 °C for 30 s, at 55 °C for 60 s, at 72 °C for 60 s). The products of amplification were evaluated in 20 g/L of agar gel by electrophoresis and stained by brom etydyne. The electrophore was visualized in VI-KS400i/Image of PC system (Syngen Biotech, USA).
The minimum detectable dose of HCV was 50 copies per mL of serum.
The sFas/sFasL concentration in serum was measured twice by quantitative sandwich enzyme immunoassay (ELISA) technique (Bender Med Systems, Austria).
Informed consent was obtained from each patient and the Bioethics Committee at the Medical University of Bia³ystok approved the study protocol.
Statistical analysis was performed by use of non-parametric Mann-Whitney U, Wilcoxon rank and Pearson tests. P < 0.05 was considered statistically significant.
Twenty-four (71%) of 34 healthy HBsAg carriers showed active HBV replication. The HBV-DNA levels exceeding 105/mL were observed in 22 (65%) HBsAg carriers. According to National Institutes of Health, such persons should be considered for antiviral treatment as chronic hepatitis B patients[4,5]. All individuals in chronic hepatitis B group presented HBV-DNA levels exceeding 105/mL.
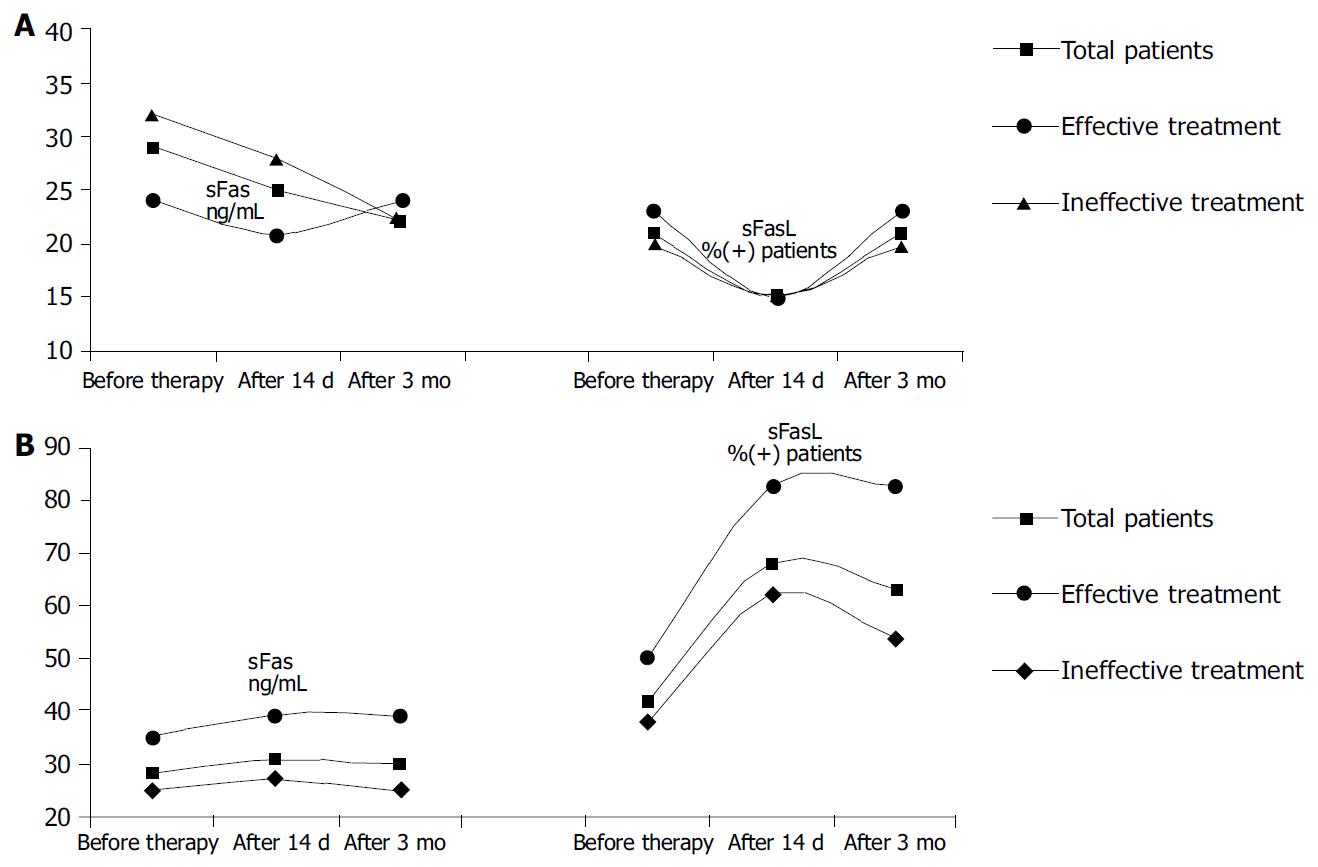
Independently from HBV-DNA levels, the concentration of sFas among HBsAg carriers and healthy persons was comparable (x = 15.4 ng/mL, x = 18.4 ng/mL, P < 0,05). Among patients with chronic HBV or HCV infection, the concentration of sFas was significantly elevated in comparison to healthy persons and healthy HBsAg carriers (x = 29.0 ng/mL, x = 28.0 ng/mL, P < 0,05), (Figure 1A). There were no statistical differences between chronic hepatitis B and C patients (Table 1).
| n | sFas-medium ng/mL | patients with sFasL (%) | sFasL-medium ng/mL | |
| Healthy | 12 | 18.4 | 0 | 0 |
| Total healthy HBsAg carriers | 34 | 15.4 | 56 | 0.9 |
| Healthy HBsAg carriers | ||||
| HBV-DNA < 104/mL or 0 | 12 | 13.6 | 58 | 0.6 |
| Healthy HBsAg carriers | ||||
| HBV-DNA > 105/mL | 22 | 16.3 | 55 | 1.0 |
| Chronic hepatitis B | 33 | 29 | 32 | 0.18 |
| Chronic hepatitis C | 19 | 28 | 68 | 1.0 |
sFasL was not detected in control group. This protein was identified more frequently in chronic hepatitis C patients than in chronic hepatitis B patients.
ALT activity reached normal values during the first three months of lamivudine treatment in all chronic hepatitis B patients (114 IU before treatment vs 32 IU after 3 mo of therapy, P < 0,001). In the same period the concentration of sFas was decreased in all patients (before treatment x = 29.0 ng/mL, after 14 d of therapy x = 25.0 ng/mL, after 3 mo of therapy x = 22.0 mg/mL). FasL expression in chronic hepatitis B group was decreased 14 d after an antiretroviral treatment and then returned to initial values.
The normalization of ALT was observed 2 wk after therapy in patients with chronic hepatitis C (102 IU before treatment vs 30 IU after 14 d of therapy). All of the chronic hepatitis C patients included in our study had undetectable HCV-RNA after 6 mo of treatment. The highest concentration of sFas was observed in chronic hepatitis C patients with virological response to antiretroviral treatment. The concentration of this protein kept increas during therapy (all HCV infected patients: before treatment x = 28.0 ng/mL, after 14 d x = 31.0 ng/mL, after 3 mo of therapy x = 30.0 mg/mL patients with HCV infection had good response: before treatment x = 35.0 ng/mL, after 14 d x = 39.0 ng/mL, after 3 mo of therapy x = 39.0 mg/mL), (Figure 1B). The number of patients with sFasL occurrence in serum was the highest before and 3 mo after treatment (83%) among all patients who were effectively treated.
Fas and FasL are “death domain”, proteins which regulate the process of apoptosis in patients with chronic inflammatory diseases, such as viral hepatitis[6-9]. The Browicz-Kupffer cells participate in the process. It stimulates synthesis of cytokines regulating biosynthesis of Fas in hepatocytes. Recent studies indicated that it was dependent on the liver or serum sFas concentration and apoptosis activity as well as hepato-cytotoxic processes in viral chronic hepatitis patients[10,11].
In this study we showed that the concentration of sFas in healthy persons and healthy HBsAg carriers was comparable and not dependant on the HBV-DNA levels. It was confirmed trough the lack of correlation between HBV-DNA levels and apoptosis. The concentration of sFas was significantly higher in patients with chronic hepatitis B infection than in healthy HBsAg carries, independent similar levels of HBV-DNA (> 105/mL).
Interestingly a large number of healthy HBsAg carriers presented sFasL in serum. The possible explanation of this fact is that HBV might stimulate expression of FasL on lymphocytes. The complex FasL and Fas could activate apoptosis and cytotoxic T lymphocytes. Activation of inflammation processes in the liver could influence FasL activity, associated with Fas and FasL complex formation. It could explain more uncommon occurrences of sFasL in patients with chronic hepatitis B than in healthy HBsAg carriers.
Hepatitis C virus core proteins could activate Fas particles expressed on hepatocytes through TNF-RI receptors stimulation. These receptors could enhance cysteine protease and subsequently interleukin-1β -converting enzyme (ICE) and FADD like ICE (FLICE-Caspase 8) synthesis. Moreover ICE and FLICE could activate nuclear protein degradation, resulting in irreversible damage of cell DNA[12-14].
However HCV could also inhibit apoptosis. HCV non-structural protein NS5A could suppress synthesis of Caspase 3, protein’s kinase A and TNF-α stimulating polymerase[15-17]. The protein’s kinase A is known as an apoptosis initiating factor.
In patients with chronic hepatitis C, insufficient apoptosis activity could cause chronic inflammation[18]. According to Di Martino et al[19] activity of apoptosis in chronic hepatitis C patients was proportional to HCV-RNA levels. Ozaslan et al[20] suggested a positive dependence on chronic hepatitis C intensity and serum sFas concentration.
Our study showed a significant increase of sFas concentration in HCV infection patients compared to healthy persons, as well as a further raise in sFas serum levels during antiviral therapy. Moreover, we observed an association between high activity of apoptosis expressed as serum sFas concentration and sFasL occurrence, and antiviral treatment effectiveness.
Increased expression of FasL on peripheral blood mononuclear cells might be caused by IFN-α apoptosis stimulation[21]. This fact is a probable explanation of the increased number of patients presenting sFasL during antiviral therapy. It may also confirm the beneficial influence of apoptosis in controlling chronic inflammation during HCV infection.
sFas serum concentration in chronic hepatitis B and C patients was comparable before treatment, but after antiviral therapy the concentration of this protein behaved in a different way. These differences underlined the diverse pathogenesis of an inflammation during chronic HBV and HCV infections. Our results indicated possible, new trends in antiviral therapy efficacy.
In conclusion, inflammation activity is associated with apoptosis in chronic hepatitis B, and there is no correlation between apoptosis and HBV-DNA concentrations. In HCV infection the effective therapy is accompanied with high activity of apoptosis, which can be a result of IFN-α activity.
Edited by Wang XL Proofread by Chen WW and Xu FM
| 1. | Wang XZ, Chen XC, Chen YX, Zhang LJ, Li D, Chen FL, Chen ZX, Chen HY, Tao QM. Overexpression of HBxAg in hepatocellular carcinoma and its relationship with Fas/FasL system. World J Gastroenterol. 2003;9:2671-2675. [PubMed] |
| 2. | Mita E, Hayashi N. [Apoptosis in human diseases: role of Fas system in liver cell injury by viral hepatitis]. Rinsho Byori. 1997;45:477-482. [PubMed] |
| 3. | Zhao J, Wang S, Xin S, Yi T, Liu P, Zhang L. [Expression of CPP32, Fas and Fas ligand and the relationship between their expression and viral antigens in chronic hepatitis]. Zhonghua Ganzangbing Zazhi. 2000;8:233-235. [PubMed] |
| 4. | Heo J, Baik TH, Kim HH, Kim GH, Kang DH, Song GA, Cho M, Yang US. Serum hepatitis B virus (HBV) DNA levels at different stages of clinical course in patients with chronic HBV infection in an endemic area. J Korean Med Sci. 2003;18:686-690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Mommeja-Marin H, Mondou E, Blum MR, Rousseau F. Serum HBV DNA as a marker of efficacy during therapy for chronic HBV infection: analysis and review of the literature. Hepatology. 2003;37:1309-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 214] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Yin XM, Ding WX. Death receptor activation-induced hepatocyte apoptosis and liver injury. Curr Mol Med. 2003;3:491-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Canbay A, Higuchi H, Bronk SF, Taniai M, Sebo TJ, Gores GJ. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology. 2002;123:1323-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 226] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Ehrmann J, Galuszková D, Ehrmann J, Krc I, Jezdinská V, Vojtések B, Murray PG, Koláo Z. Apoptosis-related proteins, BCL-2, BAX, FAS, FAS-L and PCNA in liver biopsies of patients with chronic hepatitis B virus infection. Pathol Oncol Res. 2000;6:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Lee MO, Kang HJ, Cho H, Shin EC, Park JH, Kim SJ. Hepatitis B virus X protein induced expression of the Nur77 gene. Biochem Biophys Res Commun. 2001;288:1162-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Liaw YF, Tsai SL, Chien RN, Yeh CT, Chu CM. Prednisolone priming enhances Th1 response and efficacy of subsequent lamivudine therapy in patients with chronic hepatitis B. Hepatology. 2000;32:604-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Xin S, Zhao J, Wang L. [Study on relationship between the apoptosis of hepatocytes and liver fibrosis of chronic viral hepatitis]. Zhonghua Shiyan He Linchuangbing Duxue Zazhi. 2000;14:31-33. [PubMed] |
| 12. | Hahn CS, Cho YG, Kang BS, Lester IM, Hahn YS. The HCV core protein acts as a positive regulator of fas-mediated apoptosis in a human lymphoblastoid T cell line. Virology. 2000;276:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Marusawa H, Hijikata M, Chiba T, Shimotohno K. Hepatitis C virus core protein inhibits Fas- and tumor necrosis factor alpha-mediated apoptosis via NF-kappaB activation. J Virol. 1999;73:4713-4720. [PubMed] |
| 14. | Zhu N, Ware CF, Lai MM. Hepatitis C virus core protein enhances FADD-mediated apoptosis and suppresses TRADD signaling of tumor necrosis factor receptor. Virology. 2001;283:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Ghosh AK, Majumder M, Steele R, Meyer K, Ray R, Ray RB. Hepatitis C virus NS5A protein protects against TNF-alpha mediated apoptotic cell death. Virus Res. 2000;67:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Ezelle HJ, Balachandran S, Sicheri F, Polyak SJ, Barber GN. Analyzing the mechanisms of interferon-induced apoptosis using CrmA and hepatitis C virus NS5A. Virology. 2001;281:124-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Satoh S, Hirota M, Noguchi T, Hijikata M, Handa H, Shimotohno K. Cleavage of hepatitis C virus nonstructural protein 5A by a caspase-like protease(s) in mammalian cells. Virology. 2000;270:476-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Zuckerman E, Zuckerman T, Sahar D, Streichman S, Attias D, Sabo E, Yeshurun D, Rowe J. bcl-2 and immunoglobulin gene rearrangement in patients with hepatitis C virus infection. Br J Haematol. 2001;112:364-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Di Martino V, Brenot C, Samuel D, Saurini F, Paradis V, Reynés M, Bismuth H, Féray C. Influence of liver hepatitis C virus RNA and hepatitis C virus genotype on FAS-mediated apoptosis after liver transplantation for hepatitis C. Transplantation. 2000;70:1390-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Ozaslan E, Kiliçarslan A, Simşek H, Tatar G, Kirazli S. Elevated serum soluble Fas levels in the various stages of hepatitis C virus-induced liver disease. J Int Med Res. 2003;31:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Yoneyama K, Goto T, Miura K, Mikami K, Ohshima S, Nakane K, Lin JG, Sugawara M, Nakamura N, Shirakawa K. The expression of Fas and Fas ligand, and the effects of interferon in chronic liver diseases with hepatitis C virus. Hepatol Res. 2002;24:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |