Published online Dec 15, 2004. doi: 10.3748/wjg.v10.i24.3616
Revised: April 9, 2004
Accepted: April 16, 2004
Published online: December 15, 2004
AIM: To investigate the gastroprotective effect and mechanism of amtolmetin guacyl (AMG, MED15) in mice.
METHODS: Male and female Kunming strain mice, weighing 18-22 g, were utilized in the experiment. Normal or ethanol-induced gastric mucosal damage models in mice were successfully established to investigate the gastroprotective effect and mechanism of AMG. In the experiment of gastric mucosal damage after repeated treatment with AMG, the mice were randomly divided into 5 groups: normal group, 3 AMG groups receiving (75, 150 and 300 mg/kg), and tolmetin group receiving 90 mg/kg. The mice were randomly divided into 6 groups as follows: normal group, model group, AMG groups with doses of 75, 150 and 300 mg/kg, respectively, and tolmetin group with a dose of 90 mg/kg in ethanol-induced gastric mucosal damage experiment. The severity of gastric mucosal lesions was scored from 0 to 5. Gastric tissue sections were stained with hematoxylin and eosin (HE) and examined under light microscopy. Also gastric tissue sections were stained with uranyl acetate and lead citrate, and examined under electron microscopy. In addition, nitric oxide (NO) and malondialdehyde (MDA) contents, and nitric oxide synthase (NOS) and superoxide dismutase (SOD) activities in the stomach tissue homogenates were measured by biochemical methods.
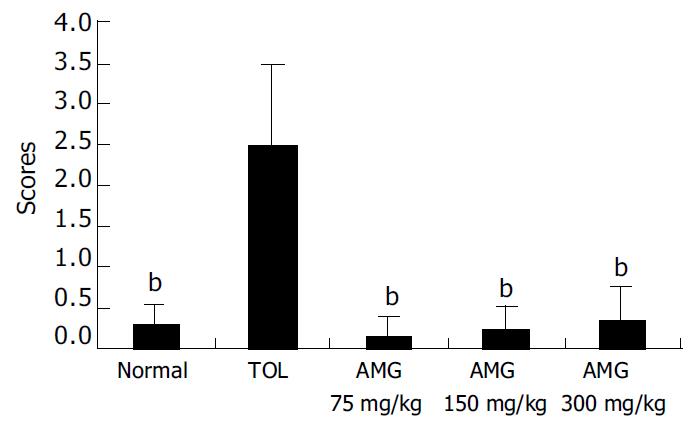
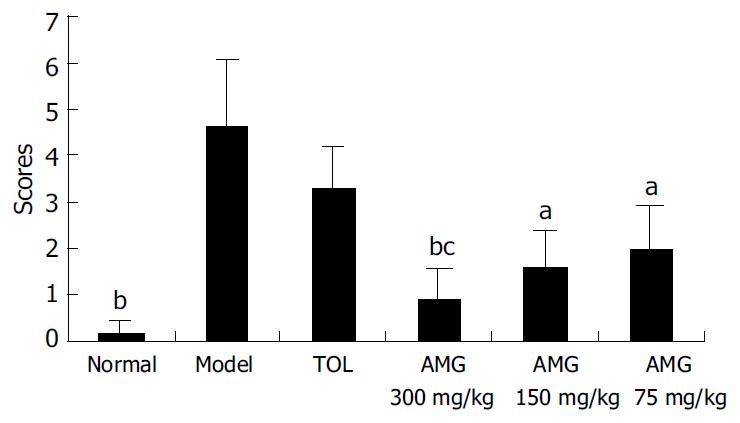
RESULTS: Repeated treatment with AMG (75, 150 and 300 mg/kg) for 7 d did not induce any appreciable mucosal damage, and the average score was not significantly different from that of normal mice. In contrast, tolmetin (90 mg/kg) produced significant gastric mucosal lesions compared with the normal group (P < 0.01). AMG (75, 150 and 300 mg/kg) significantly reduced the severity of gastric lesions induced by ethanol in a dose-dependent manner as compared with the model group (P < 0.05, AMG 75 and 150 mg/kg vs model; P < 0.01, AMG 300 mg/kg vs model). Light and electron microscopy revealed that AMG (150 and 300 mg/kg) induced minimal changes in the surface epithelium layer, without vascular congestion or leucocyte adherence. AMG (75, 150 and 300 mg/kg) demonstrated dose-dependent gastroprotective effects on mice in our study. AMG (75, 150 and 300 mg/kg) could significantly increase NO content and NOS level in the stomach homogenates of mice compared with the model group (P < 0.05, AMG 75 mg/kg and 150 mg/kg groups vs model group; P < 0.01, AMG 300 mg/kg vs model group) respectively. Moreover, AMG (150 and 300 mg/kg) not only significantly increased SOD activities but also obviously decreased the MDA content in the stomach homogenates of mice.
CONCLUSION: AMG exerts significant gastroprotective actions on mice and the involved mechanisms may be its antioxidative effect and induction of NO production.
- Citation: Li YH, Li J, Huang Y, Lü XW, Jin Y. Gastroprotective effect and mechanism of amtolmetin guacyl in mice. World J Gastroenterol 2004; 10(24): 3616-3620
- URL: https://www.wjgnet.com/1007-9327/full/v10/i24/3616.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i24.3616
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the extensively utilized medicines worldwide with antipyretic, analgesic and anti-inflammatory properties. Besides the direct stimulation, NSAIDs have some other adverse reactions to the gastrointestinal system, such as nausea, vomit, bellyache and even ulcer, perforation. All these effects are considered to be associated with the inhibition of prostaglandin (PG) synthesis in gastrointestinal system, and thereby limiting the clinical application of NSAIDs[1-7].
Amtolmetin guacyl (AMG) is a novel NSAID, and its chemical name is 2-methoxyphenyl-1-methyl-5-p-methylbenzoyl-pyrrol-2-acetamido acetate, whose metabolites are MED5 (chemical name: 1-methyl-5-p-methylbenzoyl-pyrrol-2-acetamido acetic acid) and tolmetin (TOL)[8-12]. As a novel NSAID, it has been reported that AMG possesses antipyretic, analgesic and anti-inflammatory effects in previous studies[13-20]. At the same time, it can also enhance the NOS activity of gastric mucosae, which facilitates the synthesis and release of NO so as to reduce the gastrointestinal system damage[21-23].
In this study, normal and ethanol-induced gastric mucosal lesion models in mice were established to investigate the gastroprotective effect and associated mechanisms, which could guide the rational use of medicine in clinical practice.
Male and female Kunming strain mice, weighing 18-22 g, were purchased from Animal Center of Anhui Medical University. They were housed in plastic boxes, 5 mice in each box. All mice were allowed to take food and tape water ad libitum. AMG and TOL were provided by Anhui Kelong Medicine Company. The assay kits of MDA, SOD, NOS and Coomassie brilliant blue reagents were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All experimental protocols described in this study were approved by the Ethics Review Committee for animal experimentation of anhui medical university.
The mice were equally divided into 5 groups randomly: normal group, 3 AMG groups (3 different doses) and TOL group. The mice in AMG groups intragastrically received 75, 150 or 300 mg/kg of AMG a day through an 18-gauge stainless steel animal feeding needle for 7 d prior to the experiment. Similarly, the mice in TOL group intragastrically received 90 mg/kg of TOL a day. In normal group, the mice were only fed with the same volume (0.5 mL/mouse) of carboxymethylcellulose (CMC). On the seventh day, all the mice were sacrificed by cervical dislocation and the stomachs were removed, opened along the great curvature and examined for the macroscopic evaluation of gastric mucosae. The severity of gastric mucosal lesions was examined by an experienced histologist who was unaware of the treatment conditions.
The mice were randomly divided into six groups as follows: normal group, model group, 3 AMG groups receiving 75, 150 and 300 mg/kg, respectively, and TOL group receiving 90 mg/kg, ten mice in each group. The 5 groups were intragastrically administered AMG, TOL and CMC (model group) once. One hour later, the animals received 500 mL/L ethanol (0.5 mL/mouse) intragastrically except normal group mice. One hour later, the animals were killed and the stomachs were removed. The severity of gastric mucosal lesions and histological assessments were made by an experienced histologist who was unaware of the treatment conditions.
The animals were killed, the stomachs were removed, rinsed with 5 mL of saline and immersed in 100 mL/L formalin. They were later opened along the greater curvature, for the macroscopic evaluation of gastric mucosae[21,24]. The severity of lesions was scored from 0 to 5: 0, normal; 0.5, light local reddening; 1, general reddening or small hemorrhage (< 1 mm); 2, large hemorrhage (> 1 mm); 3, small ulcer (< 2 mm); 4, large ulcer (> 2 mm); and 5, perforated ulcer. One score was assigned to each lesion. A researcher who was unaware of the treatment conditions gave the scores of gastric mucosal injury.
Light microscopy At the end of the experiment, the stomach was immediately exposed and a small strip was excised from the glandular portion, 3 mm below and parallel to the limiting ridge, so that the greater curvature was approximately located in the middle of the strip. The tissue samples were fixed in 100 mL/L formalin, 5-µm thick serial sections were taken from each block and stained with hematoxylin-eosin. The image of the sections to be examined was displayed on a color monitor by means of a videocamera attached to the microscope. An experienced histologist who was unaware of the treatment conditions made histological assessments.
Transmission electron microscopy After mice were killed, the stomachs were immediately removed, and a strip was excised in the same position as for light microscopy. Small specimens were fixed in 25 g/L glutaraldehyde liquid for 3 h at room temperature and post-fixed in 10 g/L OsO4 in 0.1 mol/L phosphate buffer (pH7.2) for 90 min at room temperature. After dehydration in a graded series of acetone and embeded in Araldite resin, semithin sections were cut for orientation. Thin sections (80 nm thick) were cut perpendicularly to the luminal surface, stained with uranyl acetate and lead citrate, and examined with a Zeiss109 electron microscope. An experienced histologist who was unaware of the treatment conditions made histological assessments.
Stomach tissue samples were weighed and homogenized in 9 g/L NaCl for the detection of SOD, MDA, NOS, NO2-. Homogenates were centrifuged at 4000 r/min for 10 min. Aliquots of the supernatants were used for studies. The assayed parameters were expressed as per mg protein, and the protein content of aliquots was determined by the method of Coomassie brilliant blue.
SOD, MDA and NOS levels in stomach tissue homogenates were assayed by using assay kits (Nanjing Jiancheng Bioengineering Institute).
NO2- concentration in stomach tissue homogenates was measured as described by Prado et al[26].
The data were analyzed by SPSS 10.0 for windows. Results were expressed as mean ± SD. Student’s t test was used for statistical analysis. P < 0.05 was considered statistically significant.
Repeated treatment with AMG (75, 150 and 300 mg/kg) for 7 d did not induce any appreciable mucosal damage, and the average score was not significantly different from that of normal mice. In contrast, TOL, the prodrug of AMG, intragastrically administered at 90 mg/kg produced significant gastric mucosal lesions compared with the normal group (P < 0.01) (Figure 1).
As showed in Figure 2, compared with that in the normal group, the average score of mice in the model group significantly increased (P < 0.01). AMG (75, 150 and 300 mg/kg) significantly reduced the severity of gastric lesions induced by ethanol in a dose-dependent manner as compared with the model group (P < 0.05, AMG 75 and 150 mg/kg vs model; P < 0.01, AMG 300 mg/kg vs model). There was no obvious difference between TOL group and model group (Figure 2).
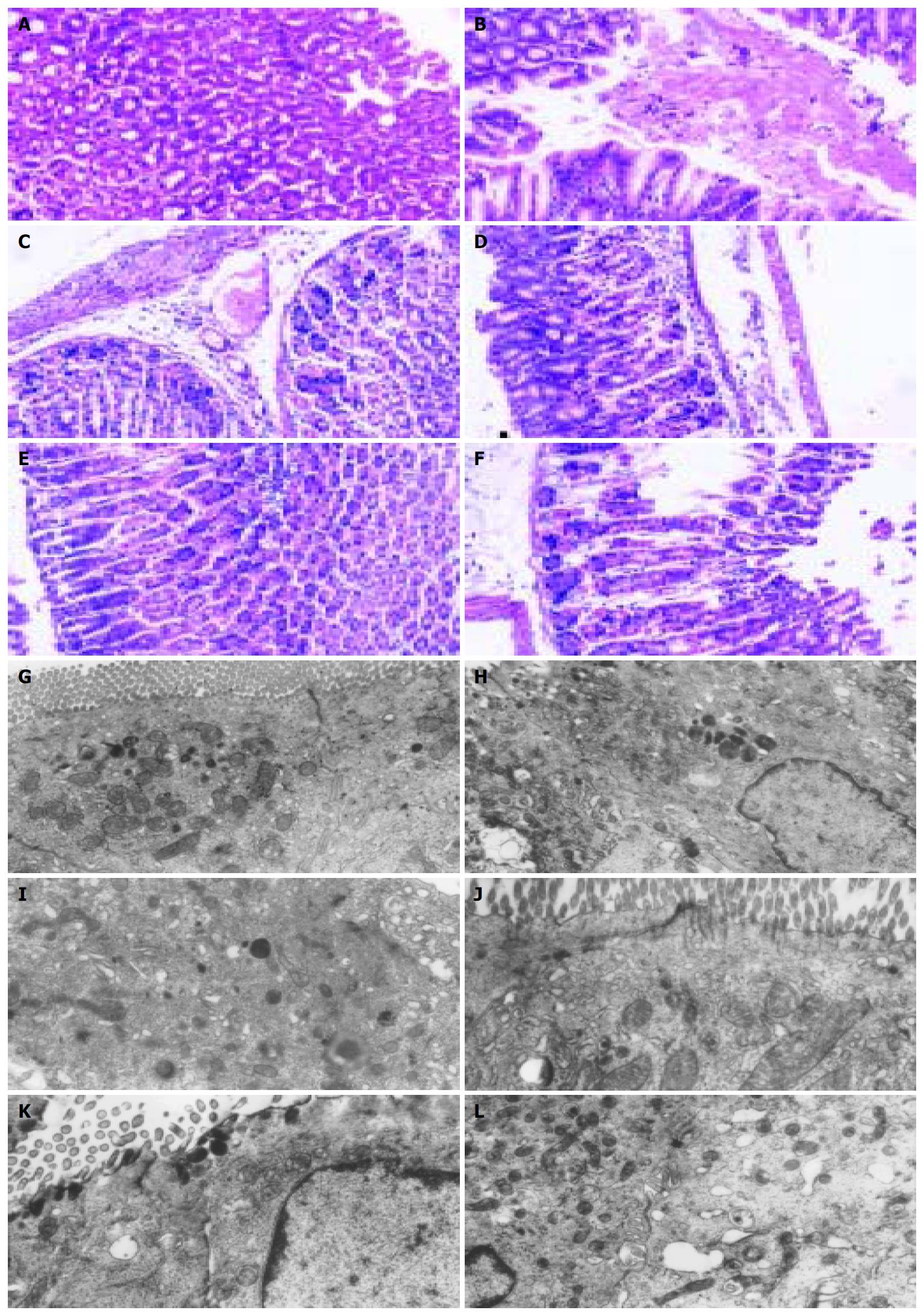
Light microscopy As shown in Figure 3 for evaluation by light microscopy, the structure of gastric mucosae was normal in normal group. The cells were well stained by HE and there was no edema or exfoliation. In contrast, a visible hemorrhagic area was found in model group. In mice treated with AMG (300 mg/kg), the epithelial and parietal cells were slightly edematous with few epithelial cells exfoliated. In the group treated with AMG (150 mg/kg), severe edema was found with inflammatory cell infiltration. In the group administered AMG (75 mg/kg), edema was more severe in epithelial and parietal cells than that in AMG (300 mg/kg) group and many epithelial cells were deciduous. Conversely, in the TOL group, together with inflammatory cell infiltration, a hemorrhagic area was observed (Figure 3A).
Transmission electron microscopy As shown in Figure 3, gastric mucosal microvilli in normal group were well arranged with no defection and shorting, while those in the model and TOL groups were mostly deciduous, broken and defective. The microvilli were almost integrated and well arranged with no defection in mice treated with AMG (300 mg/kg). In mice administered AMG (150 mg/kg), the lesions were much smaller, while the microvilli were short and small and in a bad order with obvious defection in mice treated with AMG (75 mg/kg) (Figure 3B).
Compared with those in the normal group, the NO content and NOS level were significantly decreased in the model group (P < 0.01). AMG (75, 150 and 300 mg/kg) could significantly increase the decreased NO content and NOS level compared with the model group (P < 0.05, AMG 75 mg/kg and 150 mg/kg groups vs model group; P < 0.01, AMG 300 mg/kg vs model group) respectively. The NO content and NOS level in TOL group where not evidently different from those in the model group (Table 1).
Results are shown in Table 2. Compared with that in the normal group, the SOD activity in the model group was significantly lower, but the MDA content in the model group was increased (P < 0.01). However, compared with model group, AMG (150 and 300 mg/kg) could significantly increase the SOD activity while evidently reduce the MDA level in stomach homogenates (P < 0.01, Table 2).
Gastrointestinal system lesions are resulted from the major adverse reaction of NSAIDs, which limits the wide clinical application of NSAIDs. Though the mechanism of the lesion remains unclear, some documents have proposed that these effects are related with the inhibition of PGs releasing from the mucosal epithelial cells[1,3,27]. It is known that vascular damage is considered to be the earliest process of gastric mucosal ulcer. Vasodilators, such as PGs and NO, play an important role in gastroprotection. At present ,NO, gastroprotective NSAIDs, specific cyclooxygenase-2 (COX-2) inhibitors as well as COX and 5-lipoxygenase (5-LOX) double inhibitors are used in reducing the adverse reactions of NSAIDs[28,29].
It has been demonstrated that AMG has gastroprotective properties[21-23]. Tubaro et al[30] found that repeated treatment with AMG did not induce gastric mucosa damage. Another animal experiment indicated that AMG (50-300 mg/kg ig) did not induce stomach lesions in rats, while its metabolite TOL (15-60 mg/kg ig) did in a dose-dependent manner[21]. Light and electron microscopic assessment suggested that AMG only caused very slight epithelial cell changes without vascular congestion and WBC adherence.
In our study, the 7-d treatment with AMG (75, 150 and 300 mg/kg ig) did not induce any appreciable mucosal damage, and the score was not different from that of normal group, while TOL (90 mg/kg ig) produced severe gastric mucosal lesions compared with normal group. Compared to its metabolite TOL, AMG had obvious gastroprotective effects. In ethanol-induced gastric mucosal damage model, the scores of AMG (75, 150 and 300 mg/kg ig) obviously decreased in a dose-dependent fashion. Compared to that in TOL group, the scores in AMG (300 mg/kg) group were greatly decreased. Furthermore, the scores had no obvious difference between TOL and model groups. Microscopic assessment of ethanol-induced gastric lesions showed that the degree of gastric damages in AMG (300 mg/kg) group was smaller than that in TOL and model groups, indicating the effect of AMG on gastric mucosa. Ultrastructural studies suggested that the microvilli were almost integrated and well arranged with no defection in mice treated with AMG (300 mg/kg), but those in the model and TOL groups were mostly deciduous, broken and defective.
Tubaro et al[30] found that AMG could strongly inhibit the hydrochloric acid (HCl) excretion caused by histamine so as to exert gastroprotective effects. Compared with acetylsalicylic acid, AMG (50 mg/kg ig) caused no appreciable change in basal potential difference (PD) values in gastric mucosae. AMG (100 mg/kg ig) alleviated ethanol-induced damages, increased NOS activity and stimulated NO release, and thereby showing its gastric protective effect. A research showed that the nonspecific NOS inhibitor (L-NAME 10 mg/kg sc) could reverse the effect of AMG[21]. Therefore, we concluded that the protective effect of AMG might be involved in NO release from gastric epithelial cells. In our study, AMG (75, 150 and 300 mg/kg) could significantly increased the NO content and NOS level in ethanol-induced gastric mucosal damage model group. These results suggested that the gastroprotective effect of AMG might be associated with promotion of NOS activity and induction of NO release, which is in agreement with previous reports[21,31].
Moreover, it has been reported that ethanol is capable of generating oxygen radicals, inhibiting glutathione synthesis, producing glutathione loss from tissues, increasing MDA levels and impairing antioxidative defense systems in experimental animals. Our study also showed that AMG (150 and 300 mg/kg) could sharply decrease the increased MDA level while enhance the decreased SOD activity in the models of mice, suggesting that the gastroprotective effect of AMG could at least partly contribute to the antioxidative action, which has not been reported before.
In summary, AMG has significant gastroprotective effects in mice, and its mechanism may be associated with its antioxidative effect and promotion of NO release.
Edited by Kamar M and Wang XL Proofread by Xu FM
| 1. | Wallace JL. NSAID gastroenteropathy: past, present and future. Can J Gastroenterol. 1996;10:451-459. [PubMed] |
| 2. | Yoshitani K, Kawaguchi M, Tatsumi K, Sasaoka N, Kurumatani N, Furuya H. Intravenous administration of flurbiprofen does not affect cerebral blood flow velocity and cerebral oxygenation under isoflurane and propofol anesthesia. Anesth Analg. 2004;98:471-476, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | García Rodríguez LA, Hernández-Díaz S. Risk of uncomplicated peptic ulcer among users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Am J Epidemiol. 2004;159:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Sinatra RS, Shen QJ, Halaszynski T, Luther MA, Shaheen Y. Preoperative rofecoxib oral suspension as an analgesic adjunct after lower abdominal surgery: the effects on effort-dependent pain and pulmonary function. Anesth Analg. 2004;98:135-140, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Kolesnikov YA, Wilson RS, Pasternak GW. The synergistic analgesic interactions between hydrocodone and ibuprofen. Anesth Analg. 2003;97:1721-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Fries JF, Bruce B. Rates of serious gastrointestinal events from low dose use of acetylsalicylic acid, acetaminophen, and ibuprofen in patients with osteoarthritis and rheumatoid arthritis. J Rheumatol. 2003;30:2226-2233. [PubMed] |
| 7. | Dominick KL, Dudley TK, Grambow SC, Oddone EZ, Bosworth HB. Racial differences in health care utilization among patients with osteoarthritis. J Rheumatol. 2003;30:2201-2206. [PubMed] |
| 8. | Annuziato L, di Renzo G. A bioequivalence study in man of table and capsule formulations of the nonsteroidal anti-inflammatory compound 2-methoxyphenyl 1-methyl-5-(p-methylbenzoylpyrrol)-2-acetamidoacetae. Clin Ter. 1993;142:3-10. |
| 9. | Bianchi Porro G, Montrone F, Lazzaroni M, Manzionna G, Caruso I. Clinical and gastroscopic evaluation of amtolmetin guacyl versus diclofenac in patients with rheumatoid arthritis. Ital J Gastroenterol Hepatol. 1999;31:378-385. [PubMed] |
| 10. | Arrigoni-Martelli E. Profile of activity of a new anti-inflammatory agent, ST 679 (MED 15). Drugs Exp Clin Res. 1990;16:63-66. [PubMed] |
| 11. | Mancinelli A, Bruno G, Cardace G, Morabito E, Marzo A, Arrigoni Martelli E. High-performance liquid chromatographic evaluation of Med 15 and its metabolites Med 5 and tolmetin in rat plasma. J Chromatogr. 1991;553:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Annunziato L, di Renzo G. [A bioequivalence study in man of tablet and capsule formulations of the nonsteroidal anti-inflammatory compound 2-methoxyphenyl 1-methyl-5-(p-methylbenzoylpyrrol)-2-acetamidoacetate]. Clin Ter. 1993;142:3-10. [PubMed] |
| 13. | Coruzzi G, Bertaccini G. Gastric effects of the novel non-steroidal anti-inflammatory drug amtolmetin guacyl. Nutrition. 1999;15:325-326. [PubMed] |
| 14. | Nanni G, Sarro G, Antoci G, Bianchi P, Gossetti F, Catarci M, La Pinta M, Negro P, Nolfe G. [The drug treatment of postoperative pain]. Clin Ter. 1993;142:41-46. [PubMed] |
| 15. | Nappi C, Nolfe G, La Pinta M, Colace G, Ruotolo C, Affinito P. [The treatment of postoperative pain in obstetrical-gynecologic surgery. A comparative study between ST-679 and paracetamol]. Clin Ter. 1993;142:47-52. [PubMed] |
| 16. | De Santis E, La Pinta M, Nolfe G. [An evaluation of the analgesic activity and tolerance of ST-679 in patients with pain following orthopedic and traumatological interventions]. Clin Ter. 1993;142:53-59. [PubMed] |
| 17. | Donati G, Spinazzè R. [A clinical study to determine the optimal dosage of ST-679 in the treatment of rheumatic diseases]. Clin Ter. 1993;142:19-28. [PubMed] |
| 18. | Lingetti M, Ciarimboli M, Porfido FA, Imparato L, Sorrentino GP, Garzya G, Saraceni G, Donati G, Nolfe G. [An evaluation of the therapeutic activity and tolerance of ST-679 in patients with osteoarthritis at different sites. A controlled double-blind study vs tolmetin]. Clin Ter. 1993;142:29-40. [PubMed] |
| 19. | Meloni P, Demuro G, Cara L, Garau D, Uras G, Suddu L. [Evaluation of the effectiveness and tolerability of MED 15 vs. piroxicam in patients with acute epicondylitis]. Clin Ter. 1995;146:453-456. [PubMed] |
| 20. | Alicicco E, Delfino M, Kleszczynski D. [Clinical trial comparing a new NSAID with 2 different dosages and diclofenac in patients with arthralgia in acute phase]. Clin Ter. 1995;146:595-601. [PubMed] |
| 21. | Pisano C, Grandi D, Morini G, Coppelli G, Vesci L, Lo Giudice P, Pace S, Pacifici L, Longo A, Coruzzi G. Gastrosparing effect of new antiinflammatory drug amtolmetin guacyl in the rat: involvement of nitric oxide. Dig Dis Sci. 1999;44:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Tubaro E, Belogi L, Mezzadri CM, Bettelli E. Impact on the bowel of amtolmetin guacyl, a new gastroprotective non-steroidal anti-inflammatory drug. Eur J Pharmacol. 2003;467:173-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Riezzo G, Chiloiro M, Montanaro S. Protective effect of amtolmetin guacyl versus placebo diclofenac and misoprostol in healthy volunteers evaluated as gastric electrical activity in alcohol-induced stomach damage. Dig Dis Sci. 2001;46:1797-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Xia M, Tao JY. Pathogenesis of ethanol induced gastric mu-cosal lesion in mice. Xin Xiaohuabingxue Zazhi. 1997;5:211-212. |
| 25. | Yao HW, Li J, Jin Y, Zhang YF, Li CY, Xu SY. Effect of leflunomide on immunological liver injury in mice. World J Gastroenterol. 2003;9:320-323. [PubMed] |
| 26. | Prado WA, Schiavon VF, Cunha FQ. Dual effect of local application of nitric oxide donors in a model of incision pain in rats. Eur J Pharmacol. 2002;441:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Villegas I, La Casa C, de la Lastra CA, Motilva V, Herrerías JM, Martín MJ. Mucosal damage induced by preferential COX-1 and COX-2 inhibitors: role of prostaglandins and inflammatory response. Life Sci. 2004;74:873-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Bonabello A, Galmozzi MR, Canaparo R, Isaia GC, Serpe L, Muntoni E, Zara GP. Dexibuprofen (S+-isomer ibuprofen) reduces gastric damage and improves analgesic and antiinflammatory effects in rodents. Anesth Analg. 2003;97:402-408, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Morini G, Guaita E, Lazzaretti M, Grandi D, Coruzzi G. Morphological features of rat gastric mucosa after acute and chronic treatment with amtolmetin guacyl: comparison with non-selective and COX-2-selective NSAIDs. Digestion. 2003;68:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Tubaro E, Belogi L, Mezzadri CM. The mechanism of action of amtolmetin guacyl, a new gastroprotective nonsteroidal anti-inflammatory drug. Eur J Pharmacol. 2000;387:233-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Coruzzi G, Coppelli G, Spaggiari S, Cavestro GM, Okolicsanyi L, Lo Giudice P, Pisano C, Tepperman BL. Gastroprotective effects of amtolmetin guacyl: a new non-steroidal anti-inflammatory drug that activates inducible gastric nitric oxide synthase. Dig Liver Dis. 2002;34:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |