Published online Dec 15, 2004. doi: 10.3748/wjg.v10.i24.3608
Revised: May 6, 2004
Accepted: May 13, 2004
Published online: December 15, 2004
AIM: To investigate the expression between γ-aminobutyric acid (GABA) and glutamate decarboxylase and its relation with differentiation and maturation of jejunal epithelial cells in rat jejunum.
METHODS: Immunohistochemical expression of GABA and glutamate decarboxylase (GAD, including two isoforms, GAD65 and GAD67) was investigated in rat jejunum. Meanwhile, double staining was performed with GAD65 immunohistochemistry, followed by lectin histochemistry of fluorescent wheat germ agglutinin. Furthermore, evaluation of cell kinetics in jejunum was conducted by 3H-thymidine autoradiography and immunohistochemistry using a monoclonal antibody to proliferating cell nuclear antigen (PCNA).
RESULTS: The cells showing positive immunoreactivity GABA and GAD65 were mainly distributed in the villi in rat jejunum, while jejunal epithelial cells were negative for GAD67. Positive GABA or GAD65 staining was mainly located in the cytoplasm and along the brush border of epithelial cells in the middle and upper portions. In addition, a few GABA and GAD65 strongly positive cells were scattered in the upper two thirds of jejunal villi. Double staining showed that GAD65 immunoreactivity was not found in goblet cells. 3H-thymidine-labeled nuclei were found in the lower and middle portions of jejunal crypts, which was consistent with PCNA staining. Therefore, GABA and GAD65 were expressed in a maturation or functional zone.
CONCLUSION: The characteristic expression of GABA and GAD suggests that GABA might be involved in regulation of differentiation and maturation of epithelial cells in rat jejunum.
- Citation: Wang FY, Watanabe M, Zhu RM, Maemura K. Characteristic expression of γ-aminobutyric acid and glutamate decarboxylase in rat jejunum and its relation to differentiation of epithelial cells. World J Gastroenterol 2004; 10(24): 3608-3611
- URL: https://www.wjgnet.com/1007-9327/full/v10/i24/3608.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i24.3608
γ-aminobutyric acid (GABA), originally identified as the principal inhibitory neurotransmitter in the mammalian brain, has been demonstrated to be biologically active in different tissues throughout the body[1-3]. In developing embryoes, GABA was verified to play an important role in the morphogenesis and maturation of many tissues outside the nervous system[4,5]. Our previous study indicated that GABA and glutamate decarboxylase (GAD, including two isoforms, GAD65 and GAD67) were expressed in chondrocytes on the epiphyseal growth plate of rats, and mainly localized in the maturation zone, rather than the reserve zone or proliferating zone[6]. This suggests that GABA might play certain functional roles in the differentiation of chondrocytes during growth of the skeleton.
Recently, GABA and GAD have been proved to be increased in colorectal carcinoma tissues by both biochemical and immunohistochemical methods[7,8]. However, the distribution patterns of GABA and GAD in growth zones of the intestinal epithelium have not been clarified. Therefore, the present study was designed to detect the expression of GABA and GAD in the growth zones of rat jejunum, with an attempt to elucidate the relationship between GABA expression and differentiation and maturation of intestinal epithelial cells.
Rabbit anti-GAD65 polyclonal antibody was purchased from Sigma (Sigma Co. St. Louis, MO, USA). Rabbit anti-GABA and anti-GAD67 polyclonal antibodies were acquired from Chemicon International Inc.(Temecula, CA, USA). Mouse anti-PCNA monoclonal antibody was obtained from Medical and Biological Laboratories Co. (Nagoya, Japan). Alexa FluoTM 488 goat anti-rabbit IgG (H + L) and Alexa FluorR 594 wheat germ agglutinin (WGA) conjugates were acquired from Molecular Probes (Eugene, OR, USA). Biotin-conjugated anti-mouse immunoglobulin polyclonal antibody was purchased from Pharmingen International (San Diego, CA, USA). 3H-thymidine was obtained from PerkinElmer Life Science Inc. ([6-3H]¯thymidine, specific activity: 528 GBq/mmol, Boston, MA, USA).
Male Wistar rats (4-6 wk, Nihon Clea, Osaka, Japan), weighing 80-100 g, were caged under controlled conditions of light (lights on 06:00-18:00 h) and temperature (23 °C). The rats were given food and water ad libitum. The Ethics Review Committee for Animal Experimentation of Osaka Medical College approved the experimental protocol.
The animals (n = 5) were deeply anesthetized with pentobarbital (50 mg/kg body weight), and then fixed by transcardial perfusion with 40 g/L paraformaldehyde in Ringer’s solution. After whole body fixation, segments of jejunum (2 cm from Treitz’s ligament) were excised and immersed in cold 40 g/L paraformaldehyde in phosphate buffered saline (PBS, pH7.2) at 4 °C overnight. For light microscopy study, tissues were soaked overnight in 300 g/L sucrose in PBS, and longitudinal cryostat 5 μm thick sections were cut on a freezing microtome (Leica CM 3050, Nusloch, Germany).
Immunohistochemical study was performed with polyclonal antibodies against GABA, GAD65, and GAD67. The final dilution for these antibodies was 1:800, 1:1000, and 1:1000, respectively. With all antibodies, a two-step indirect immunohistochemical method was used. Cryostat sections were fixed with ice-cold acetone, incubated with 100 mL/L normal goat serum at room temperature for 60 min, and then incubated with primary antibodies overnight at 4 °C. Incubation with primary antisera was followed by Alexa FluoTM488-labeled goat anti-rabbit immunoglobulins. The secondary antibodies were diluted to 1:250 in PBS prior to use, incubated for 60 min at room temperature in darkness, and washed three times with 0.01 mol/L PBS. Sections were finally mounted with MO2 Crystal/Mount (Cosmo Bio, Tokyo, Japan) and preserved at 4 °C in a dark refrigerator. Primary antibodies were replaced by PBS for the negative controls. None of the controls revealed any specific signal.
Sections were first applied to immunohistochemical staining for GAD65 as aforementioned. After reaction with the second antibody and a brief wash in PBS, sections were further incubated with Alexa FluorR 594 WGA at room temperature for 60 min in darkness, and washed with in 0.01 mol/L PBS three times and mounted with MO2 Crystal/Mount.
Rats (n = 2) were injected intraperitoneally at 10:00 a.m with 100 μCi (3.7 MBq) 3H-thymidine. After 90 min, the rats were anesthetized and fixed by intracardial perfusion with 25 g/L glutaraldehyde. Samples of jejunum were taken as aforementioned, and 5 μm thick paraffin sections were prepared regularly. Autoradiography[9] was performed as follows: Tissue sections were deparaffinized and dipped into NR-M2 emulsion (Konica Co. Tokyo, Japan) that was diluted with an equal volume of distilled water containing 10 g/L glycerin. After 10 d of exposure in a dark refrigerator, the sections were developed at 20 °C for 8 min in Kodak D-19 diluted with an equal volume of distilled water, terminated in 10 g/L acetic acid for 1 min, and fixed in 300 g/L sodium thiosulphate solution at 20 °C for 8 min. Finally, the sections were lightly counterstained with hematoxylin.
After three washes with PBS, endogenous peroxides were blocked in 10 g/L hydrogen peroxide in methanol for 30 min at room temperature. For antigen retrieval, the sections were treated with 1 g/L pepsin in 0.01 mol/L HCl. Non-specific binding sites were blocked with 40 g/L bovine serum albumin. Subsequently, anti-PCNA was diluted to 1:100 and reacted with tissue specimens at 4 °C overnight. The sections were then washed three times with PBS, and incubated with biotinylated secondary antibody at room temperature for 60 min. Finally, immunohistochemical staining was performed using the avidin-biotin-peroxidase complex (Vectastain ABC kit, Burlingame, CA, USA). Diaminobenzidine was used as a chromogen, and the sections were counterstained with hematoxylin.
For the convenience of description, the crypts of jejunum were divided into the lower, middle and upper portions, while the villi as the basal, middle and top portions. PCNA immunostaining was observed with a Nikon light microscope equipped with a digital camera (PDMC Ie, Polaroid Co., MA, USA). Fluorescence observation was performed using a confocal laser scanning microscopy (Radiance 2000, Bio-Rad Laboratories, CA, USA) equipped with an argon laser. The laser scanning differential interference contrast (DIC) and confocal mode with an argon laser at 488 nm and/or 590 nm were used. 3H-thymidine autographs were observed with a confocal laser microscope (Carl Zeiss LS10, Germany)[9]. 3H-thymidine labeling index or PCNA labeling index was calculated as the percentage of positive cells of the total cells by counting 10 different crypts[10].
Welch’s t test was used for the comparison of PCNA labeling index and 3H-thymidine labeling index.
Radioactivity was located at the nuclei of cells at the lower and middle portions of the jejunal crypts, while the upper crypts and the whole villi were negative (Figure 1). The 3H -thymidine labeling index was 30% ± 6%.
Strong PCNA staining was detected in the lower portion of the jejunal crypts, while epithelial cells in the villi were almost negative (Figure 2). PCNA labeling index was 57% ± 8%, which was significantly higher than 3H -thymidine labeling index (P < 0.01, Welch’s t test).
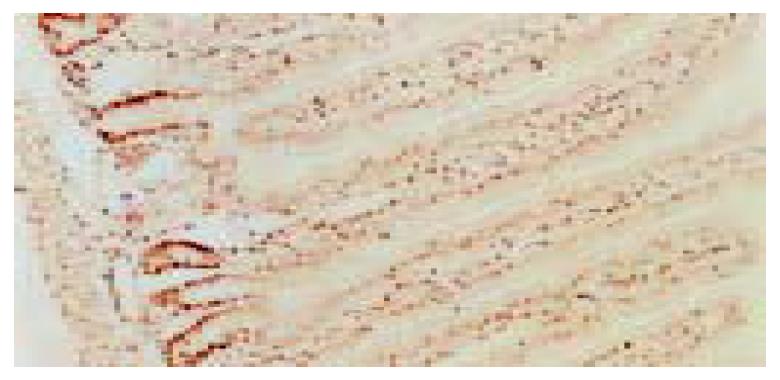
GABA immunoreactive cells were distributed in the whole villi of rat jejunum. Strongly positive staining was mainly located in the cytoplasm and along the brush border of epithelial cells in the middle and upper portions (Figure 3).
GAD65 immunopositive cells were distributed in the middle and upper portions of jejunal villi. Strongly positive staining of GAD65 was mainly localized along the brush border of enterocytes (Figure 4). In addition, a few strongly positive cells had no brush border, which were scattered in the middle or upper portion of jejunal villi. GAD67 was negative in jejunal epithelial cells.
The distribution of GABA and GAD65 immunopositive cells, in comparison with PCNA, is shown in Table 1. GABA and GAD65 were mainly localized in the place where PCNA was negative or weak positive. That is to say, GABA and GAD65 were distributed in the maturation zone and functional zone, rather than in the proliferating zone or stem cells of the jejunal epithelium.
| Crypt | Villus | |||||
| Lower | Middle | Upper | Basal | Middle | Top | |
| PCNA | ++ | +++ | ± | ± | ± | - |
| GABA | ± | - | + | + | ++ | ++ |
| GAD65 | ± | - | - | ± | ++ | ++ |
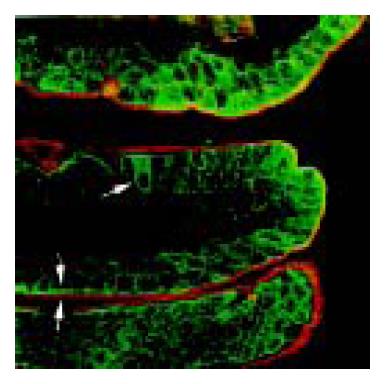
Goblet cells were demonstrated by fluorescent WGA staining, while mature absorptive cells were characterized by a well-developed brush border that was positively stained for GAD65 in the jejunum. The GAD65 strongly positive cells in jejunal villi were negative for WGA (Figure 4). Pre-epithelial mucous layer was also stained by fluorescent WGA.
The replacement and cell kinetics in murine intestines have been established for decades. The proliferation sites are existed in the lower or middle portion of the small intestinal crypt and in the lower half crypt of the large intestine in rats[11,12]. Bartkova et al[13] suggested that intestinal epithelium could be divided into four compartments, namely, stem cells at the base, proliferating zone, maturation zone, and functional zone near the luminal surface.
Cell kinetics has been examined traditionally by 3H-thymidine as a marker for S phase cells. PCNA is an evolutionarily highly conserved acidic nuclear protein, which can function as an auxiliary protein for DNA polymerase δ[14]. It has been proved that PCNA expression is maximal during S phase of the cell cycle, and PCNA mRNA normally accumulates only in proliferating cells[15]. Consequently, PCNA has been found to be a useful marker in immunohistochemical analysis of cell kinetics[16].
In this study, the growth zones of jejunum were demonstrated by PCNA immunohistochemistry and 3H-thymidine auotoradiography. The proliferating zone was consisted of the lower and middle portions of the crypt in the jejunum. Our results also indicated that 3H-thymidine auotoradiography was more specific for marking S-phase cells than PCNA immunohistochemistry, as PCNA labeling index was significantly higher than 3H-thymidine labeling index. Meanwhile, GABA and GAD65 immunoreactive cells were distributed in jejunal villi. In other words, GABA and GAD were found in maturation and function zones other than in proliferating zone in rat jejunum.
The characteristic distribution patterns of GABA and GAD in the intestinal epithelium remain unknown. Gilon et al[4] first reported their research about the possible role of GABA and cell differentiation. Their results documented the appearance of GABA in the developing pancreas and duodenum just prior to the termination of rapid growth and maturation of these tissues. These results are similar to the earlier findings in the developing brain[17]. Our previous study also demonstrated that GABA and GAD were mainly localized in the hypertrophic zones rather than in the proliferating zone in rat epiphyseal growth plate chondrocytes[6]. Recently, Kaita et al[18] reported that ciprofloxacin significantly increased the hepatic regenerative activity in animal models of alcohol-induced liver diseases. The results of PCNA staining showed an enhanced hepatic regeneration in the ciprofloxacin-treated group at 60 h (saline, 13.4% ± 3.7%; ciprofloxacin, 47.4% ± 7.3%; and putrescine, 8.4% ± 2.8% positively stained hepatocytes). Our results also showed that the intensity of immunoreactive GABA and GAD65 on well-differentiated cells was stronger than that on proliferating cells. Based on these results, we presume that GABA should be involved in the regulation of differentiation and maturation of epithelial cells. On the other hand, the characteristic distribution of GABA and GAD65 also indicates that GABA might have some inhibitory effects on epithelial proliferation in rat jejunum.
The intestine has been recognized as a “diffuse endocrine” system, where a variety of endocrine cells produce different peptide hormones. A number of studies have demonstrated that GABA exists in endocrine cells in the gastrointestinal tract[19-21]. This study showed that there were some strong GABA and GAD65 immunoreactive cells in jejunal villi. It is well known that stem cells in intestinal epithelium give rise to four different kinds of cells: enterocytes (absorptive cells), goblet cells, enteroendocrine cells, and Paneth cells. Mature enterocytes are characterized by the presence of a brush border at their apical surface, while Paneth cells are localized in the lower crypts[22]. Goblet cells in rat jejunum were clearly demonstrated by lectin histochemistry, because of the specific binding of WGA to sugar residues in mucin within the cells[23]. Double staining showed that GAD65 strongly positive cells were neither goblet cells nor mature absorptive cells. We believe that these cells are enteroendocrine cells that can synthesize GABA from glutamic acid. Therefore, it seems reasonable to assume that GABA might be related to the endocrine function of the small intestine.
GABA is known to be synthesized principally from glutamic acid via single enzymatic catalysis of GAD. Two isoforms of mammalian GAD with a predicted molecular weight of 65300 (GAD65) and 66600 (GAD67) are highly conserved, but derived from separated genes[24]. The phylogenetic tree study indicated that the multiplicity of mammalian GAD in central nervous system might have developed some 500 million years before, when the widespread of gene duplications occurred in vertebrates[25]. Though these isoforms could catalyze the same biochemical reaction, they have been proved to have a different distribution and may have different functions in peripheral as well as the nervous system[26]. Our result showed that GAD65 was positive, while GAD67 was negative in rat jejunum. This difference further supported the multiplicity of GAD in mammal non-neural tissues[27].
It should be noted that the distribution of GABA in rat jejunum was not exactly parallel to that of GAD65. In the basal third of jejunal villi, GABA was found to be moderately positive, while GAD65 was weakly positive. GABA in these cells might not be synthesized from glutamic acid via GAD. That is to say, GABA in some intestinal epithelial cells might have other synthetic routes. Putrescine route is a well-known alternative pathway of GABA synthesis in gastrointestinal tract[28]. Apart from GABA formation, putrescine can be utilized for the biosynthesis of polyamines, such as spermidine and spermine,which have been proved to be involved in the control of cell proliferation[29,30]. Further research is necessary to elucidate the metabolic routes and functional roles of GABA-polyamine system in epithelial cells of the intestine.
In conclusion, GABA and GAD65 are mainly expressed in the maturation or functional zone in jejunal epithelium of rats. This characteristic expression suggests that GABA may be involved in the regulation of differentiation and maturation of epithelial cells.
Edited by Kumar M and Wang XL Proofread by Xu FM
| 1. | Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H. GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol. 2002;213:1-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 396] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 2. | Fujimura S, Shimakage H, Tanioka H, Yoshida M, Suzuki-Kusaba M, Hisa H, Satoh S. Effects of GABA on noradrenaline release and vasoconstriction induced by renal nerve stimulation in isolated perfused rat kidney. Br J Pharmacol. 1999;127:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Azuma H, Inamoto T, Sakamoto T, Kiyama S, Ubai T, Shinohara Y, Maemura K, Tsuji M, Segawa N, Masuda H. Gamma-aminobutyric acid as a promoting factor of cancer metastasis; induction of matrix metalloproteinase production is potentially its underlying mechanism. Cancer Res. 2003;63:8090-8096. [PubMed] |
| 4. | Gilon P, Reusens-Billen B, Remacle C, Janssens de Varebeke P, Pauwels G, Hoet JJ. Localization of high-affinity GABA uptake and GABA content in the rat duodenum during development. Cell Tissue Res. 1987;249:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Gilon P, Mallefet J, De Vriendt C, Pauwels S, Geffard M, Campistron G, Remacle C. Immunocytochemical and autoradiographic studies of the endocrine cells interacting with GABA in the rat stomach. Histochemistry. 1990;93:645-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Tamayama T, Kanbara K, Maemura K, Kuno M, Watanabe M. Localization of GABA, GAD65 and GAD67 in rat epiphyseal growth plate chondrocytes. Acta Histochem Cytochem. 2001;34:201-206. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Kleinrok Z, Matuszek M, Jesipowicz J, Matuszek B, Opolski A, Radzikowski C. GABA content and GAD activity in colon tumors taken from patients with colon cancer or from xenografted human colon cancer cells growing as s.c. tumors in athymic nu/nu mice. J Physiol Pharmacol. 1998;49:303-310. [PubMed] |
| 8. | Maemura K, Yamauchi H, Hayasaki H, Kanbara K, Tamayama T, Hirata I, Watanabe M. Gamma-amino-butyric acid immunoreactivity in intramucosal colonic tumors. J Gastroenterol Hepatol. 2003;18:1089-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Kuroda E, Watanabe M, Tamayama T, Shimada M. Autoradiographic distribution of radioactivity from (14)C-GABA in the mouse. Microsc Res Tech. 2000;48:116-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Yamada K, Yoshitake K, Sato M, Ahnen DJ. Proliferating cell nuclear antigen expression in normal, preneoplastic, and neoplastic colonic epithelium of the rat. Gastroenterology. 1992;103:160-167. [PubMed] |
| 11. | Podolsky DK. Regulation of intestinal epithelial proliferation: a few answers, many questions. Am J Physiol. 1993;264:G179-G186. [PubMed] |
| 12. | Thompson JS, Saxena SK, Sharp JG. Regulation of intestinal regeneration: new insights. Microsc Res Tech. 2000;51:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Bartkova J, Thullberg M, Slezak P, Jaramillo E, Rubio C, Thomassen LH, Bartek J. Aberrant expression of G1-phase cell cycle regulators in flat and exophytic adenomas of the human colon. Gastroenterology. 2001;120:1680-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987;326:515-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1328] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 15. | Shpitz B, Bomstein Y, Mekori Y, Cohen R, Kaufman Z, Grankin M, Bernheim J. Proliferating cell nuclear antigen as a marker of cell kinetics in aberrant crypt foci, hyperplastic polyps, adenomas, and adenocarcinomas of the human colon. Am J Surg. 1997;174:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Chen H, Wang LD, Guo M, Gao SG, Guo HQ, Fan ZM, Li JL. Alterations of p53 and PCNA in cancer and adjacent tissues from concurrent carcinomas of the esophagus and gastric cardia in the same patient in Linzhou, a high incidence area for esophageal cancer in northern China. World J Gastroenterol. 2003;9:16-21. [PubMed] |
| 17. | Watanabe M, Shimada M, Watanabe H, Nakanishi M. Amino acid content in several brain regions of the active and hibernating frog, Rana esculenta. Comp Biochem Physiol B. 1990;97:605-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Kaita KD, Assy N, Gauthier T, Zhang M, Meyers AF, Minuk GY. The beneficial effects of ciprofloxacin on survival and hepatic regenerative activity in a rat model of fulminant hepatic failure. Hepatology. 1998;27:533-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Krantis A, Tufts K, Nichols K, Morris GP. [3H]GABA uptake and GABA localization in mucosal endocrine cells of the rat stomach and colon. J Auton Nerv Syst. 1994;47:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Davanger S, Hjelle OP, Babaie E, Larsson LI, Hougaard D, Storm-Mathisen J, Ottersen OP. Colocalization of gamma-aminobutyrate and gastrin in the rat antrum: an immunocytochemical and in situ hybridization study. Gastroenterology. 1994;107:137-148. [PubMed] |
| 21. | Krantis A, Mattar K, Glasgow I. Rat gastroduodenal motility in vivo: interaction of GABA and VIP in control of spontaneous relaxations. Am J Physiol. 1998;275:G897-G903. [PubMed] |
| 22. | Kong SE, Heel K, McCauley R, Hall J. The role of enterocytes in gut dysfunction. Pathol Res Pract. 1998;194:741-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Bryk SG, Sgambati E, Gheri Bryk G. Lectin histochemistry of goblet cell sugar residues in the gut of the chick embryo and of the newborn. Tissue Cell. 1999;31:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Ahman AK, Wågberg F, Mattsson MO. Two glutamate decarboxylase forms corresponding to the mammalian GAD65 and GAD67 are expressed during development of the chick telencephalon. Eur J Neurosci. 1996;8:2111-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Bosma PT, Blázquez M, Collins MA, Bishop JD, Drouin G, Priede IG, Docherty K, Trudeau VL. Multiplicity of glutamic acid decarboxylases (GAD) in vertebrates: molecular phylogeny and evidence for a new GAD paralog. Mol Biol Evol. 1999;16:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Cram DS, Faulkner-Jones B, Kun J, Harrison LC. Glutamic acid decarboxylase-67 (GAD67): expression relative to GAD65 in human islets and mapping of autoantibody epitopes. Endocrinology. 1995;136:1111-1119. [PubMed] |
| 27. | Katarova Z, Sekerková G, Prodan S, Mugnaini E, Szabó G. Domain-restricted expression of two glutamic acid decarboxylase genes in midgestation mouse embryos. J Comp Neurol. 2000;424:607-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Tillakaratne NJ, Medina-Kauwe L, Gibson KM. gamma-Aminobutyric acid (GABA) metabolism in mammalian neural and nonneural tissues. Comp Biochem Physiol A Physiol. 1995;112:247-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 138] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Hardt J, Larsson LI, Hougaard DM. Immunocytochemical evidence suggesting that diamine oxidase catalyzes biosynthesis of gamma-aminobutyric acid in antropyloric gastrin cells. J Histochem Cytochem. 2000;48:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Höpfner M, Berger A, Fölsch UR, Löser C. Effects of insulin-like growth factor I on growth and polyamine metabolism in various organs in rats. Digestion. 2002;65:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |