Published online Dec 1, 2004. doi: 10.3748/wjg.v10.i23.3464
Revised: November 20, 2003
Accepted: December 6, 2003
Published online: December 1, 2004
AIM: To examine the serological response of patients with upper gastrointestinal diseases and Helicobocter pylori (H pylori) infection to two H pylori outer membrane proteins (OMPs) (Mr18000 and Mr26000) acquired by gene recombinant technique, and to determine the diagnostic significance of serological tests derived from these OMPs.
METHODS: Recombinant vectors encoding the two H pylori OMPs were used to transform and express in BL21 (DE3) E.coli. After purification with Ni2+-NTA agarose resin, colloid gold kits were prepared with purified recombinant proteins to detect H pylori infection and H pylori-associated diseases by the immunity-marker technology. We selected 150 patients with H pylori infection and digestive symptoms without previous treatment, including chronic gastritis (n = 60), duodenal ulcer (n = 30), gastric ulcer (n = 30), and gastric cancer (n = 30). As controls, 33 H pylori-negative healthy volunteers were also recruited. Serum samples were collected from all subjects, and the antibodies to specific proteins of H pylori were tested with the colloid gold test kits. The sensitivity, specificity and accuracy of the colloid gold tests were evaluated, by using the combination of standard diagnostic methods (13C urea breath test and bacteria culture) and classic enzyme-linked immunosorbent assay (ELISA) as reference.
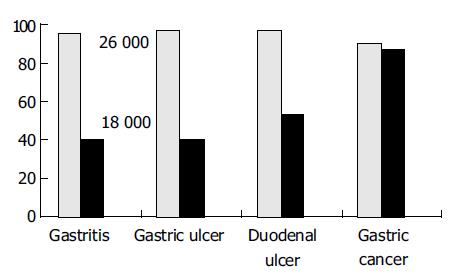
RESULTS: After purification with Ni2+-NTA agarose resin, the purity of recombinant fusion proteins was about 95%. The recombinant fusion proteins were recognized by the specific monoclonal antibodies against the two H pylori OMPs, as demonstrated by the ELISA. Of the 150 serum samples from patients infected with H pylori 141 (94.0%) responded positively to the recombinant protein with Mr26000, while the seropositive rates were 95.0%, 96.7%, 96.7% and 90.0% for patients with H pylori-associated chronic gastritis, duodenal ulcer, gastric ulcer, and gastric cancer respectively. The sensitivity, specificity, and accuracy of the colloid gold kit with Mr26000 protein were 94.0%, 97.0%, and 94.5%, respectively. Compared with the classic ELISA, bacteria culture and 13C urea breath test results in detecting H pylori-infection, there was no significant difference (P > 0.05). For the colloid gold kit with Mr18000, the seropositive rates were 52.0%, 40.0%, 40.0%, 53.3% and 86.7%, respectively, in H pylori-infected patients, and those with H pylori-associated chronic gastritis, duodenal ulcer, gastric ulcer, and gastric cancer. There was a significant difference (P < 0.05) in seropositivity between patient with gastric cancer (86.7%) and those with other diseases (43.3%).
CONCLUSION: The two colloid gold kits derived from the recombinant OMPs are useful tools either for detecting H pylori infection, or for, predicting H pylori-associated gastric malignancy.
-
Citation: Jiang Z, Huang AL, Tao XH, Wang PL. Diagnosis of
Helicobacter pylori infection and diseases associated with Helicobacter pylori byHelicobacter pylori outer membrane proteins. World J Gastroenterol 2004; 10(23): 3464-3469 - URL: https://www.wjgnet.com/1007-9327/full/v10/i23/3464.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i23.3464
Since the initial report of an unidentified curved bacillus located on the gastric epithelium of patients with chronic active gastritis, the discovery of Helicobocter pylori (H pylori) and its association with a number of gastrointestinal diseases has revolutionized gastroenterology. It has attracted the interest of scholars in gastroenterology and microbiology. Infection of gastric mucosa with H pylori could be found in approximately 50% of the world population[1], its association with peptic ulcer disease, chronic gastritis, mucosa-associated lymphoid tissue lymphoma, and gastric adenocarcinoma has been well documented over the past two decades[2-19]. Moreover it was also found in some extradigestive diseases[20-36]. The direct evidence of carcinogenesis was recently demonstrated in an animal model[37,38], so this organism has been recently categorized as a class I carcinogenetic factor by the World Health Organization. It is obviously important to detect and eradicate H pylori infection.
The routine detecting methods including invasive and non-invasive tests have differences in sensitivity and specificity, each with their indication and characteristics in clinical practice[39-48]. Because of great quantity of serum samples especially in epidemiological studies, enzyme linked immunosorbent assay (ELISA) is the widely used test[49-52]. Antigens used in ELISA are divided into three kinds. The first is the total cell of microbacteria sonicated by ultrasonic wave, which is easy to be confused with Helicobacter, campylobacter, or a diverse range of other bacteria and incur to intercourse response with each other in detecting H pylori infection. The second is the partly purified antigens, with greatly increased specificity and decreased intercourse response, but H pylori could not be cultured in great quantity without special apparatus and conditions. The last is the recombinant purified antigen by gene recombinant technique. To date, many genes of OMP of H pylori have been amplified by scholars with polymerase chain reaction from H pylori chromosomes and inserted into the compatible sites of expression vectors by using T4 DNA ligase. Moreover recombinant vectors could be expressed in E.coli[53-62], but a few reports are available on lower-molecular-mass OMP application to the detection of H pylori infection and diagnose H pylori-associated diseases. In order to acquire a great amount of purified 18000, 26000 OMP of H pylori, we constructed recombinant vectors containing genes encoding with Mr18000, 26000 OMP of H pylori expressed in E.coli respectively and identified the antigenicity of expressed products[63]. So we prepared the colloid gold kits with purified recombinant proteins by antigen-antibody reaction and gold-marked technique to determine whether they were capable of detecting H pylori infection and H pylori-associated diseases.
Well-characterized strains of BL21/pET32a (+) /Omp26, BL21/ pET32a (+) /Omp18 were constructed, expressed and identified by the Department of Microbiology. The expression fusion proteins were recognized by the corresponding monoclonal antibody with Mr18 000, 26 000 OMP of H pylori and the animal’s serum immunized with recombinant fusion proteins respectively. After purification using Ni2+-NTA agarose resin columniation, the purity of recombinant fusion proteins was about 95%. 13C urea breath test was purchased from Headway Company, Ni2+-NTA agarose resin columniation was obtained from QIAGEN Company, ultrasonic liquid (50 mmol/L NaH2PO4, 300 mmol/L NaCl, PH 7.0), abluent (50 mmol/L phosphate, 300 mmol/L NaCl, 20 mmol/L imidazole, pH 7.80) and lavation (50 mmol/L phosphate, 300 mmol/L NaCl, 250 mmol/L imidazole, pH 7.80) were provided by the Institute of Viral Hepatitis of Chongqing University of Medical Sciences.
Sera were collected from 150 patients with gastrointestinal symptoms and H pylori infection in the Outpatient Clinic of the Gastroenterology Department of the University Hospital during Jan. 2002 to Dec. 2002 including 60 cases of gastritis, 30 cases of gastric ulcer, 30 cases of duodenal ulcer and 30 cases of gastric cancer diagnosed by gastroscopy. Sera from 31 healthy volunteers without H pylori infection were collected as control. All testee were forbidden to take H2-antagonists, corticosteriods, proton pump inhibitors and antibiotics within 4 wk.
Single bacterial colonies (BL21/pET32a (+) /Omp18, BL21/ pET32a (+) /Omp26) were picked and cultured respectively in 2 mL LB broth containing 100 mg/L of ampicillin, at 300 r/min at 37 °C overnight. On the next day, BL21 E.coli strains containing recombinant plasmids were grown until mid-log phase (Absorbence at 600 nm = 0.5 to 1.0), and then induced to express recombinant fusion proteins in 100 mL LB by adding 1 mmol/L IPTG for 4 h. Following induction, bacteria were harvested by centrifugation at 12000 r/min for 15 min, and stored at -20 °C for SDS-PAGE analysis.
Due to C end of recombinant fusion antigens with six histidines, recombinant fusion antigens were purified with Ni2+-NTA agarose resin. Briefly, 500 mL of cultivated bacteria suspension was prepared, centrifuged, resuspended with the buffer liquid (50 mmol/L phosphate, 300 mmol/L NaCl, pH 7.0), and sonicated by ultrasonic wave with the energy of 600 W × 35% for 40 min, and ultracentrifuged for 15 min at 10000 g at 4 °C. The sonicated recombinant fusion antigens were purified using Ni2+-NTA agarose resin with abluent (50 mmol/L phosphate, 300 mmol/L NaCl, 20 mmol/L imidazole, pH 7.80) and lavation (50 mmol/L phosphate, 300 mmol/L NaCl, 250 mmol/L imidazole, pH 7.80), and quantified. The antigenicities of expressed recombinant fusion proteins were determined by immunoblotting. Following electrophoretic transfer of SDS-PAGE-separated (150 g/L acrylamide) recombinant fusion proteins to 0.45 µm pore size PVDF membrane, and after a 30-min wash in tris-saline blotting buffer, antigen-impregnated PVDF strips were incubated with the sera from patients infected with H pylori and anti-Omp18 or anti-Omp26 antibody for 2 h at RT. After washed, the proteins were detected by incubating the strips in alkaline phosphatase-conjugated goat anti-man IgG and alkaline phosphatase-conjugated goat anti-mice IgG antibody for 1 h at RT.
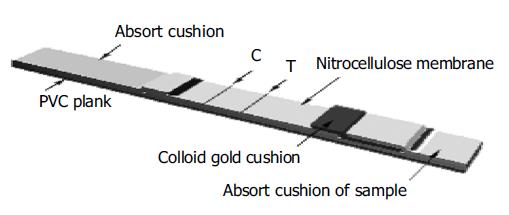
With gold marker and special antigen-antibody reaction technique, H pylori infection could be detected using the antigen-antibody-antigen method. The recombinant fusion proteins were impregnated in nitrocellulose membrane (NC) as a detecting strip, the recombinant fusion proteins were marked with gold as a colored reagent by which the special antibody of patient serum to H pylori could be detected in seconds. The criteria of the test were as follows: Negative (-): antigen-impregnated NC membrane was not recognized by patient serum, non-special antibody to H pylori in patient serum, so there was only an aubergine strip in mass-control district, and not in detecting district simultaneously. Positive (+): antigen-impregnated NC membrane was recognized by patient serum, special antibody to H pylori in patient serum. In mass-control and detecting district, there were aubergine strips. If there was no aubergine strip in mass-control district, errors might occur in experiment course. The colloid gold test paper is shown in Figure 1.
The sensitivity, specificity and accuracy of the colloid gold kits were evaluated on the basis of the serum ELISA results taken as reference with combined of standard diagnostic methods (13C urea breath test, bacteria culture as the gold standard). Patients were defined as H pylori infection if one out of two validated tests of 13C urea breath test and culture was positive, and as non-H pylori infection if two tests were negative. Patients infected with H pylori were determined as false negative if the colloid gold kits were negative; patients without H pylori infection were determined as false positive if the colloid gold kits were positive. Based on the above results, the applied value of the colloid gold kit in clinical practice was evaluated.
After pET32a (+) /Omp18 and pET32a (+) /Omp26 were transfected into BL21 E.coli strains, the strains with high expressions of fusion proteins were selected and grown respectively until mid-log phase (Absorbence at 600 nm = 0.4 to 0.6), and then induced to express recombinant fusion proteins by adding of 1 mmol/L IPTG for 4 h. Following induction, bacteria were harvested by centrifugation at 12000 g for 5 min, resuspended in protein-buffer and seethed for 5 min. Their molecular mass was Mr38000 and 46000 respectively by 150 g/L SDS-PAGE gel analysis. After the recombinant bacteria were sonicated by ultrasonic wave and ultracentrifuged (10000 g, 15 min, 4 °C), the levels of soluble fusion proteins in the supernatant were about 18.96% and 26.38% of total cellular protein respectively. After purification by Ni2+-NTA agarose resin columniation, the purity was about 95%. Recombinant fusion proteins were all recognized by the corresponding monoclonal antibody with Mr18 000, 26000 OMP of H pylori and the animal’s serum immunized with recombinant fusion proteins respectively. The results showed recombinant fusion proteins could provide excellent antigenicity.
Sera from 150 patients with gastrointestinal symptoms and H pylori infection including 60 cases of gastritis, 30 cases of gastric ulcer, 30 cases of duodenal ulcer and 30 cases of gastric cancer examined by gastroscopies, and sera from 33 healthy volunteers without H pylori infection were assayed using the colloid gold kits with Mr18000, 26000 respectively (Table 1, Figure 2). The results were as follows. Ninety-four percent of patients infected with H pylori showed response to recombinant protein with Mr26000, while 95%, 96.7%, 96.7% and 90.0% of patients with H pylori-infected chronic gastritis, gastric ulcer, duodenal ulcer, and gastric cancer showed responses (Table 2). There was no significant difference between the conventional examined methods and the colloid gold kits (P > 0.05), indicating that the prevalence of infection diagnosed by both methods was similar. To specific recombinant protein with Mr18000, 52.0% of patients showed response, while 40.0%, 40.0%, 53.3% and 86.7% of patients with H pylori-infected chronic gastritis, gastric ulcer, duodenal ulcer, and gastric cancer respectively showed responses (Table 3). Moreover there was a significant difference (P < 0.05) in the detecting rates of H pylori infection between patients with gastric cancer (86.7 %) and those with other diseases (43.3%). Based on the classic ELISA, bacteria culture and 13C urea breath test results, the sensitivity, specificity, and accuracy of the colloid gold kit with Mr26000 protein were 94.0%, 97.0%, and 94.5%, respectively.
| Methods | Testee | Positive | Negative | False positive | False negative |
| Routine methods | 183 | 144 | 32 | 1 | 6 |
| Mr26000 protein | 183 | 141 | 32 | 1 | 9 |
| Mr18000 protein | 183 | 78 | 33 | 0 | 72 |
| Disease | Patients | yr | Positive | Negative |
| Gastritis | 60 | 50.3 ± 15.9 | 57 | 3 |
| Gastric ulcer | 30 | 57.3 ± 13.2 | 29 | 1 |
| Duodenal ulcer | 30 | 46.5 ± 14.2 | 29 | 1 |
| Gastric cancer | 30 | 64.7 ± 17.4 | 27 | 3 |
| Disease | Patients | yr | Positive | Negative |
| Gastritis | 60 | 50.3 ± 15.9 | 24 | 36 |
| Gastric ulcer | 30 | 57.3 ± 13.2 | 12 | 18 |
| Duodenal ulcer | 30 | 46.5 ± 14.2 | 16 | 14 |
| Gastric cancer | 30 | 64.7 ± 17.4 | 26 | 4 |
Mr18000, 26000 OMP of H pylori are commonly expressed in all H pylori strains examined so far. Furthermore, no cross-reaction has been shown when antibodies (polyclonal and monoclonal) to low-molecular outer membrane proteins were used to screen closely related species of Helicobacteria, campylobacteria, or a diverse range of other bacteria[64]. In our study, H pylori 18000, 26000 OMP were successfully expressed in E.coli with good antigenicity. With marked gold and special antigen-antibody reaction technique, H pylori infection could be detected by the antigen-antibody-antigen method. Recombinant fusion proteins were impregnated in NC membrane as a detecting strip, and marked with gold as a colored reagent by which the special antibody to H pylori could be determined in seconds. At first the recombinant proteins were prepared in large quantities, purified and regulated. Due to the diameter size of grained gold would affect directly the result of test, the diameter of grained gold was adjusted to 40-60 nm, at the same time the concentration of antibody was adjusted in order to acquire steady and reliable products marked with gold. A test detecting IgG antibodies to H pylori was thus constructed.
Sera were collected from 150 patients with gastrointestinal symptoms and H pylori infection and from 33 healthy volunteers without H pylori infection used as controls during one year. The detecting results using colloid gold kit were as follows. Ninety-four percent of patients infected with H pylori showed response to recombinant protein with Mr26000, 95.0%, 96.7%, 96.7% and 90.0% of patients with H pylori-infected chronic gastritis, gastric ulcer, duodenal ulcer, and gastric cancer, showed responses to specific proteins with Mr26000 respectively, There was no significant difference between the routine examined methods and colloid gold kits (P > 0.05). 52.0% of patients infected with H pylori showed response to recombinant protein with Mr18000, while 40.0%, 40.0%, 53.3% and 86.7% of patients with H pylori-infected chronic gastritis, gastric ulcer, duodenal ulcer, and gastric cancer respectively, showed responses to specific proteins with Mr18000, moreover there was a significant difference (P < 0.05) in the detecting rates of H pylori infection between gastric cancer (86.7 %) and other diseases (43.3%). The results showed that colloid gold kits with Mr26000 proteins of H pylori could be used as a conventional examination method. Based on the classic ELISA, bacteria culture and 13C urea breath test results, the sensitivity, specificity, and accuracy of the rapid test kit with Mr26000 protein were 94.0%, 97.0%, and 94.5%, respectively, and a significant association was found between the serologic response to Mr18000 OMP antigen and malignant outcome of H pylori infection. The two colloid gold kits with Mr26000, 18000 proteins of H pylori, could be used to detect H pylori infection and H pylori-associated diseases, and to predict the risk of peptic ulcer or malignancy. The results mentioned above were consistent with those reported[65-67].
All strains of H pylori could express low-molecular-mass OMP, which stimulates the body to produce corresponding antibodies, moreover the antibodies produced are related to the corresponding molecular size of antigens and immuno-status of the body. Decrease of the antibodies was associated with the corresponding molecular size of the antibodies. So the results showed that the responses of patients infected with H pylori to recombinant proteins with Mr26000 were stronger (94.0%) than that (52.0%) to recombinant proteins with Mr18000, while the growth of gastric cancer was associated with much more factors and stages. All pathogenic factors may act on the pre-carcinoma stage alone or together with each other in the model of chronic gastritis-atrophic gastritis-intestinal metaplasia-atypical hyperplasia-gastric cancer. Chua et al[68] compared the seroprevalence of antibodies with various H pylori antigens in Singaporeans with gastric adenocarcinoma and the normal Singaporean population using both conventional immunoglobulin (Ig) G ELISA and Western blot immunoassay, and found that strains of H pylori including antigens with Mr19500 and seronegative antigens with Mr35000 could provide their potential for carcinogenesis. Immunoreactive species-specific Mr19500 OMP of H pylori is actually Lpp20, while its actual molecular mass is 18000 OMP. So H pylori strains based on carcinogenic potential could provide a basis for selective surveillance and eradication therapy. Low-molecular-mass OMP could lead to the 12th gene mutation of C-Ha-ras, and amplify p21 protein expressed by ras gene and c-met protein could also be overexpressed. Therefore the detecting rate of low-molecular-weight OMP of H pylori in gastric cancer is higher than other OMP. In our study, the detecting rate of Mr26000 OMP of H pylori in gastric cancer was similar to that of Mr18000 OMP. The results showed low-molecular-weight OMP could be used, not only in vaccine target candidates, but also in detection of gastric cancer in highly risk patients with H pylori infection. In a nutshell, the colloid gold kit constructed with Mr18000, 26000 OMP of H pylori could detect anti-H pylori IgG-antibody. Compared with other methods, this method not only provides a rapid, simple and painless test, but also gives a reliable specificity, especially in diagnosis of gastrointestinal tumors. The colloid gold kit based on marked gold and special antigen-antibody reaction technique is a rapid diagnostic kit, only a few minutes are required to complete an assay, and no special instruments are needed. Compared with other immunoassay techniques, the colloid gold kit has following advantages. It is based on the antigen-antibody reaction on membranes, its detecting time is much shorter than ELISA, at the same time colloid gold is red in color, so there is no need to add other colored reagents, moreover, the products marked with gold are more stable than those marked with enzyme. The colloid gold kit evaluated in our study enables a simple, rapid, noninvasive, and accurate diagnosis of H pylori infection, and is an ideal test method for screening patients with gastrointestinal tumors.
Edited by Wang XL and Xu JY Proofread by Xu FM
| 1. | Michetti P, Kreiss C, Kotloff KL, Porta N, Blanco JL, Bachmann D, Herranz M, Saldinger PF, Corthésy-Theulaz I, Losonsky G. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology. 1999;116:804-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 231] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 2. | Hiyama T, Haruma K, Kitadai Y, Masuda H, Miyamoto M, Ito M, Kamada T, Tanaka S, Uemura N, Yoshihara M. Clinicopathological features of gastric mucosa-associated lymphoid tissue lymphoma: a comparison with diffuse large B-cell lymphoma without a mucosa-associated lymphoid tissue lymphoma component. J Gastroenterol Hepatol. 2001;16:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Nakamura S, Matsumoto T, Suekane H, Takeshita M, Hizawa K, Kawasaki M, Yao T, Tsuneyoshi M, Iida M, Fujishima M. Predictive value of endoscopic ultrasonography for regression of gastric low grade and high grade MALT lymphomas after eradication of Helicobacter pylori. Gut. 2001;48:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3182] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 5. | Kate V, Ananthakrishnan N, Badrinath S. Effect of Helicobacter pylori eradication on the ulcer recurrence rate after simple closure of perforated duodenal ulcer: retrospective and prospective randomized controlled studies. Br J Surg. 2001;88:1054-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Xue FB, Xu YY, Wan Y, Pan BR, Ren J, Fan DM. Association of H. pylori infection with gastric carcinoma: a Meta analysis. World J Gastroenterol. 2001;7:801-804. [PubMed] |
| 7. | Peng ZS, Liang ZC, Liu MC, Ouang NT. Studies on gastric epithelial cell proliferation and apoptosis in Hp associated gas-tric ulcer. Shijie Huaren Xiaohua Zazhi. 1999;7:218-219. |
| 8. | Xiao SD, Liu WZ. Current statue in treatment of Hp infection. Shijie Huaren Xiaohua Zazhi. 1999;7:3-4. |
| 9. | Meyer JM, Silliman NP, Dixon CA, Siepman NY, Sugg JE, Hopkins RJ. Helicobacter pylori and early duodenal ulcer status post-treatment: a review. Helicobacter. 2001;6:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Casella G, Buda CA, Maisano R, Schiavo M, Perego D, Baldini V. Complete regression of primary gastric MALT-lymphoma after double eradication Helicobacter pylori therapy: role and importance of endoscopic ultrasonography. Anticancer Res. 2001;21:1499-1502. [PubMed] |
| 11. | Hurenkamp GJ, Grundmeijer HG, Van Der Ende A, Tytgat GN, Assendelft WJ, Van Der Hulst RW. Arrest of chronic acid suppressant drug use after successful Helicobacter pylori eradication in patients with peptic ulcer disease: a six-month follow-up study. Aliment Pharmacol Ther. 2001;15:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Guo CQ, Wang YP, Liu GY, Ma SW, Ding GY, Li LC. Study on Helicobacter pylori infection and p53, c-erbB-2 gene expression in carcinogenesis of gastric mucosa. Shijie Huaren Xiaohua Zazhi. 1999;7:313-315. |
| 13. | Hiyama T, Haruma K, Kitadai Y, Masuda H, Miyamoto M, Ito M, Kamada T, Tanaka S, Uemura N, Yoshihara M. Clinicopathological features of gastric mucosa-associated lymphoid tissue lymphoma: a comparison with diffuse large B-cell lymphoma without a mucosa-associated lymphoid tissue lymphoma component. J Gastroenterol Hepatol. 2001;16:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Hu PJ. Hp and gastric cancer: challenge in the research. Shijie Huaren Xiaohua Zazhi. 1999;7:1-2. |
| 15. | Quan J, Fan XG. Progress in experimental research of Helicobacter pylori infection and gastric cancinoma. Shijie Huaren Xiaohua Zazhi. 1999;7:1068-1069. |
| 16. | Delchier JC, Lamarque D, Levy M, Tkoub EM, Copie-Bergman C, Deforges L, Chaumette MT, Haioun C. Helicobacter pylori and gastric lymphoma: high seroprevalence of CagA in diffuse large B-cell lymphoma but not in low-grade lymphoma of mucosa-associated lymphoid tissue type. Am J Gastroenterol. 2001;96:2324-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Morgner A, Miehlke S, Fischbach W, Schmitt W, Müller-Hermelink H, Greiner A, Thiede C, Schetelig J, Neubauer A, Stolte M. Complete remission of primary high-grade B-cell gastric lymphoma after cure of Helicobacter pylori infection. J Clin Oncol. 2001;19:2041-2048. [PubMed] |
| 18. | Zhang XQ, Lin SR. Progress in research on the relationship between Hp and stomach cancer. Shijie Huaren Xiaohua Zazhi. 2000;8:206-207. |
| 19. | Hua JS. Effect of Hp: cell proliferation and apoptosis on stom-ach cancer. Shijie Huaren Xiaohua Zazhi. 1999;9:647-648. |
| 20. | Armitage GC. Periodontal infections and cardiovascular disease--how strong is the association? Oral Dis. 2000;6:335-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Tsai CJ, Huang TY. Relation of Helicobacter pylori infection and angiographically demonstrated coronary artery disease. Dig Dis Sci. 2000;45:1227-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Gocyk W, Nikliński T, Olechnowicz H, Duda A, Bielański W, Konturek PC, Konturek SJ. Helicobacter pylori, gastrin and cyclooxygenase-2 in lung cancer. Med Sci Monit. 2000;6:1085-1092. [PubMed] |
| 23. | Baysoy G, Ertem D, Ademoğlu E, Kotiloğlu E, Keskin S, Pehlivanoğlu E. Gastric histopathology, iron status and iron deficiency anemia in children with Helicobacter pylori infection. J Pediatr Gastroenterol Nutr. 2004;38:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Caselli M, Zaffoni E, Ruina M, Sartori S, Trevisani L, Ciaccia A, Alvisi V, Fabbri L, Papi A. Helicobacter pylori and chronic bronchitis. Scand J Gastroenterol. 1999;34:828-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Daudén E, Jiménez-Alonso I, García-Díez A. Helicobacter pylori and idiopathic chronic urticaria. Int J Dermatol. 2000;39:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Ojetti V, Armuzzi A, De Luca A, Nucera E, Franceschi F, Candelli M, Zannoni GF, Danese S, Di Caro S, Vastola M. Helicobacter pylori infection affects eosinophilic cationic protein in the gastric juice of patients with idiopathic chronic urticaria. Int Arch Allergy Immunol. 2001;125:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Vainio E, Huovinen S, Liutu M, Uksila J, Leino R. Peptic ulcer and Helicobacter pylori in patients with lichen planus. Acta Derm Venereol. 2000;80:427-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Szlachcic A, Sliwowski Z, Karczewska E, Bielański W, Pytko-Polonczyk J, Konturek SJ. Helicobacter pylori and its eradication in rosacea. J Physiol Pharmacol. 1999;50:777-786. [PubMed] |
| 29. | Avci O, Ellidokuz E, Simşek I, Büyükgebiz B, Güneş AT. Helicobacter pylori and Behçet's disease. Dermatology. 1999;199:140-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Yazawa N, Fujimoto M, Kikuchi K, Kubo M, Ihn H, Sato S, Tamaki T, Tamaki K. High seroprevalence of Helicobacter pylori infection in patients with systemic sclerosis: association with esophageal involvement. J Rheumatol. 1998;25:650-653. [PubMed] |
| 31. | Emilia G, Longo G, Luppi M, Gandini G, Morselli M, Ferrara L, Amarri S, Cagossi K, Torelli G. Helicobacter pylori eradication can induce platelet recovery in idiopathic thrombocytopenic purpura. Blood. 2001;97:812-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 162] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 32. | Parkinson AJ, Gold BD, Bulkow L, Wainwright RB, Swaminathan B, Khanna B, Petersen KM, Fitzgerald MA. High prevalence of Helicobacter pylori in the Alaska native population and association with low serum ferritin levels in young adults. Clin Diagn Lab Immunol. 2000;7:885-888. [PubMed] |
| 33. | Konno M, Muraoka S, Takahashi M, Imai T. Iron-deficiency anemia associated with Helicobacter pylori gastritis. J Pediatr Gastroenterol Nutr. 2000;31:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Annibale B, Lahner E, Bordi C, Martino G, Caruana P, Grossi C, Negrini R, Delle Fave G. Role of Helicobacter pylori infection in pernicious anaemia. Dig Liver Dis. 2000;32:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Choe YH, Kwon YS, Jung MK, Kang SK, Hwang TS, Hong YC. Helicobacter pylori-associated iron-deficiency anemia in adolescent female athletes. J Pediatr. 2001;139:100-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Kaptan K, Beyan C, Ural AU, Cetin T, Avcu F, Gülşen M, Finci R, Yalçín A. Helicobacter pylori--is it a novel causative agent in Vitamin B12 deficiency? Arch Intern Med. 2000;160:1349-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 169] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 37. | Nozaki K, Shimizu N, Inada K, Tsukamoto T, Inoue M, Kumagai T, Sugiyama A, Mizoshita T, Kaminishi M, Tatematsu M. Synergistic promoting effects of Helicobacter pylori infection and high-salt diet on gastric carcinogenesis in Mongolian gerbils. Jpn J Cancer Res. 2002;93:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Crabtree JE, Court M, Aboshkiwa MA, Jeremy AH, Dixon MF, Robinson PA. Gastric mucosal cytokine and epithelial cell responses to Helicobacter pylori infection in Mongolian gerbils. J Pathol. 2004;202:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Chua TS, Fock KM, Teo EK, Ng TM. Validation of 13C-urea breathtest for the diagnosis of Helicobacter pylori infection in the Singapore population. Singapore Med J. 2002;43:408-411. [PubMed] |
| 40. | Kawai T, Kawakami K, Kudo T, Ogiahara S, Handa Y, Moriyasu F. A new serum antibody test kit (E plate) for evaluation of Helicobacter pylori eradication. Intern Med. 2002;41:780-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Day AS, Sherman PM. Accuracy of office-based immunoassays for the diagnosis of Helicobacter pylori infection in children. Helicobacter. 2002;7:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Oyedeji KS, Smith SI, Arigbabu AO, Coker AO, Ndububa DA, Agbakwuru EA, Atoyebi OA. Use of direct Gram stain of stomach biopsy as a rapid screening method for detection of Helicobacter pylori from peptic ulcer and gastritis patients. J Basic Microbiol. 2002;42:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 43. | Wong WM, Wong BC, Tang VS, Lai KC, Yuen ST, Leung SY, Hu WH, Lam SK. An evaluation of the PyloriTek test for the diagnosis of Helicobacter pylori infection in Chinese patients before and after eradication therapy. J Gastroenterol Hepatol. 2001;16:976-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Kakinoki K, Takemori Y, Noda Y. [Efficacy of the urine antibody test for detection of Helicobacter pylori: comparison with serum antibody tests]. Nihon Shokakibyo Gakkai Zasshi. 2001;98:935-941. [PubMed] |
| 45. | Fujisawa T, Kaneko T, Kumagai T, Akamatsu T, Katsuyama T, Kiyosawa K, Tachikawa T, Kosaka O, Machikawa F. Evaluation of urinary rapid test for Helicobacter pylori in general practice. J Clin Lab Anal. 2001;15:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Miwa H, Akamatsu S, Tachikawa T, Sogabe T, Ohtaka K, Nagahara A, Sugiyama Y, Sato N. On-site diagnosis of H. pylori infection by urine. Diagn Microbiol Infect Dis. 2001;39:95-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Yamamoto S, Uemura N, Okamoto S, Yamaguchi S, Mashiba H, Tachikawa T. A new rapid test for detecting anti-Helicobacter pylori antibody excreted into urine. Helicobacter. 2000;5:160-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Ladas SD, Malamou H, Giota G, Varzakakos I, Kitsanta P, Georgopoulos S, Spiliadi C, Raptis SA. Prospective evaluation of a whole-blood antibody test (FlexPack HP) for in-office diagnosis of Helicobacter pylori infection in untreated patients. Eur J Gastroenterol Hepatol. 2000;12:727-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Gisbert JP, Cruzado AI, Cabrera MM, Carpio D, Benito LM, Pérez Poveda JJ, Valbuena M, Cantero J, Pajares JM. ["Rapid" serology for the diagnosis of Helicobacter pylori infection. Evaluation of its accuracy compared with a gold-standard and its concordance with "classic" serology]. Gastroenterol Hepatol. 2000;23:159-164. [PubMed] |
| 50. | Xia HH, Kalantar JS, Wyatt JM, Adams S, Cheung K, Eslick GD, Talley NJ. High sensitivity and specificity of a laboratory-based serological test, pylori DTect ELISA, for detection of Helicobacter pylori infection. Diagn Microbiol Infect Dis. 2000;36:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Miwa H, Hirose M, Kikuchi S, Terai T, Iwazaki R, Kobayashi O, Takei Y, Ogihara T, Sato N. How useful is the detection kit for antibody to Helicobacter pylori in urine (URINELISA) in clinical practice? Am J Gastroenterol. 1999;94:3460-3463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | García-Díaz E, Castro-Fernández M, Romero-Gómez M, Vargas-Romero J. The effectiveness of (IgG-ELISA) serology as an alternative diagnostic method for detecting Helicobacter pylori infection in patients with gastro-intestinal bleeding due to gastro-duodenal ulcer. Rev Esp Enferm Dig. 2002;94:725-736. [PubMed] |
| 53. | Liang SH, Mao YF, Yan J. [Cloning, expression and identification of flaB gene from a clinical isolate of Helicobacter pylori]. Zhejiang Daxue Xuebao Yixueban. 2003;32:13-16. [PubMed] |
| 54. | Mao YF, Yan J, Li LW. [Cloning, expression and identification of hpaA gene from a clinical isolate of Helicobacter pylori]. Zhejiang Daxue Xuebao Yixueban. 2003;32:9-12. [PubMed] |
| 55. | Liu X, Hu J, Zhang X, Fan D. Oral immunization of mice with attenuated Salmonella typhimurium expressing Helicobacter pylori urease B subunit. Chin Med J (Engl). 2002;115:1513-1516. [PubMed] |
| 56. | Londoño-Arcila P, Freeman D, Kleanthous H, O'Dowd AM, Lewis S, Turner AK, Rees EL, Tibbitts TJ, Greenwood J, Monath TP. Attenuated Salmonella enterica serovar Typhi expressing urease effectively immunizes mice against Helicobacter pylori challenge as part of a heterologous mucosal priming-parenteral boosting vaccination regimen. Infect Immun. 2002;70:5096-5106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Reiche N, Jung A, Brabletz T, Vater T, Kirchner T, Faller G. Generation and characterization of human monoclonal scFv antibodies against Helicobacter pylori antigens. Infect Immun. 2002;70:4158-4164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Miyashita M, Joh T, Watanabe K, Todoroki I, Seno K, Ohara H, Nomura T, Miyata M, Kasugai K, Tochikubo K. Immune responses in mice to intranasal and intracutaneous administration of a DNA vaccine encoding Helicobacter pylori-catalase. Vaccine. 2002;20:2336-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Liao W, Chen M, Zhu S. [Construction of attenuated Salmonella typhimurium vaccine strain expressing Helicobacter pylori catalase and observation on its protective immunity]. Zhonghua Yixue Zazhi. 2001;81:613-616. [PubMed] |
| 60. | Koesling J, Lucas B, Develioglou L, Aebischer T, Meyer TF. Vaccination of mice with live recombinant Salmonella typhimurium aroA against H. pylori: parameters associated with prophylactic and therapeutic vaccine efficacy. Vaccine. 2001;20:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Jiang Z, Pu D, Huang AL, Tao XH, Wang PL. [Construction, expression and antigenic study of bivalent vaccine candidate with 26,000 OMP and heat short protein A of human Helicobacter pylori]. Zhonghua Yixue Zazhi. 2003;83:862-867. [PubMed] |
| 62. | Bumann D, Metzger WG, Mansouri E, Palme O, Wendland M, Hurwitz R, Haas G, Aebischer T, von Specht BU, Meyer TF. Safety and immunogenicity of live recombinant Salmonella enterica serovar Typhi Ty21a expressing urease A and B from Helicobacter pylori in human volunteers. Vaccine. 2001;20:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Jiang Z, Huang A, Wang P. [Gene cloning and expression of outer membrane protein of Helicobacter pylori]. Zhonghua Yixue Zazhi. 2001;81:1416-1419. [PubMed] |
| 64. | Keenan J, Oliaro J, Domigan N, Potter H, Aitken G, Allardyce R, Roake J. Immune response to an 18-kilodalton outer membrane antigen identifies lipoprotein 20 as a Helicobacter pylori vaccine candidate. Infect Immun. 2000;68:3337-3343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 65. | Raymond J, Sauvestre C, Kalach N, Bergeret M, Dupont C. Immunoblotting and serology for diagnosis of Helicobacter pylori infection in children. Pediatr Infect Dis J. 2000;19:118-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Todoroki I, Joh T, Watanabe K, Miyashita M, Seno K, Nomura T, Ohara H, Yokoyama Y, Tochikubo K, Itoh M. Suppressive effects of DNA vaccines encoding heat shock protein on Helicobacter pylori-induced gastritis in mice. Biochem Biophys Res Commun. 2000;277:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 67. | Shiesh SC, Sheu BS, Yang HB, Tsao HJ, Lin XZ. Serologic response to lower-molecular-weight proteins of H. pylori is related to clinical outcome of H. pylori infection in Taiwan. Dig Dis Sci. 2000;45:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Chua TS, Fock KM, Chan YH, Dhamodaran S, Sim CS, Ng TM, Teo EK. Seroreactivity to 19.5-kDa antigen in combination with absence of seroreactivity to 35-kDa antigen is associated with an increased risk of gastric adenocarcinoma. Helicobacter. 2002;7:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |