Published online Dec 1, 2004. doi: 10.3748/wjg.v10.i23.3455
Revised: February 20, 2004
Accepted: February 24, 2004
Published online: December 1, 2004
AIM: To investigate the inhibitory effect of phosphorothioate anti-sense oligodeoxynucleotides (PASODN) on colorectal cancer LS-174T cells in vitro and the mechanism of inhibition of telomerase activity in these cells.
METHODS: PASODN were used to infect LS-174T cells and block human telomerase RNA (hTR) through anti-sense technology. The inhibitory effect of PASODN was evaluated by colony-forming inhibition assay and growth curve. Changes of telomerase activity in LS-174T cells were detected by polymerase chain reaction-enzyme-linked immunosorbent assay (PCR-ELISA), and the level of apoptosis was analyzed by flow cytometry (FCM) assay.
RESULTS: PASODN showed a dose and time-dependent inhibition of cell proliferation. The optimal dosage of PASODN was 10 μmol/L. The colony-forming efficiency was 10.3% in PASODN group after 10 d, whereas that in phosphorothioate mis-sense oligodeoxynucleotides (PMSODN) group with the same concentration and in PBS group (blank control) was 49.1% and 50.7%, respectively. PCR-ELISA results indicated that telomerase activity in the PASODN group was obviously inhibited in comparison with in the control groups (P < 0.01, t = 3.317 and 3.241, t0.01(20) = 2.845). Meanwhile, before the number of cells was decreased, the morphological changes were observed in the cells of PASODN group. The cells in PASODN group showed the apoptotic peak at 72 h after infection, whereas the control group did not show.
CONCLUSION: Specific sequence oligonucleotides can inhibit telomerase activity and lead to cell apoptosis, suggesting a novel treatment strategy for malignant tumors induced by telomerase.
- Citation: Wang XS, Wang K, Li X, Fu SB. Effects of phosphorothioate anti-sense oligodeoxynucleotides on colorectal cancer cell growth and telomerase activity. World J Gastroenterol 2004; 10(23): 3455-3458
- URL: https://www.wjgnet.com/1007-9327/full/v10/i23/3455.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i23.3455
Telomerase, a ribonucleic acid-protein complex, adds hexameric repeats of 5’-TTAGGG-3’ to the ends of telomeres to compensate for the progressive loss in malignant tumor cells[1,2]. Because of its preferential expression in malignant tumors and lack of expression in normal tissues[3-5], the idea that telomerase is a potential target of cancer therapy has been widely accepted[6,7]. From the theoretical point of view, telomerase-associated therapeutic approach to telomerase-positive tumors is the anti-telomerase cancer therapy that directly inhibits telomerase activity, resulting in apoptotic cell death or growth arrest. Two major components of the telomerase holoenzyme complex: human telomerase RNA (hTR) and catalytic subunit (human telomerase reverse transcriptase, hTERT), are considered as the therapeutic targets[8]. Since the growth of majority, or even parts of malignant tumors depends on telomerase, the strategy of anti-telomrease cancer therapy is necessary and important. In this study, we investigated the inhibitory effect of PASODN on colorectal cancer cells.
Human colorectal cancer cell line LS-174T was obtained from Shanghai Institute of Cell Biology (Shanghai, China). Oligonucleotides were synthesized in Shenggong Company (Shanghai, China). The sequences of oligonucleotides were as follows: PASODN: 5’-TTAGGG-3’, and PMSODN: 5’-TGTGAG-3’. Telomerase PCR-ELISA kit (No. 1854666) was purchased from Roche Company.
Colorectal cancer LS-174T cells were cultured in RPMI 1640 (GIBCO, UK) supplemented with 100 mL/L charcoal-stripped FCS (GIBCO, UK), 500 units/mL penicillin and 0.1 μg/mL streptomycin (SIGMA, USA) at 37 °C with a humidified atmosphere containing 50 mL/L CO2. The medium was changed every 48 h. Cell cycle was 12 h approximately. Cells in logarithmic phase were used in all experiments.
The LS-174T cells (100 cells/mL in each well) were transferred to 24-well plates and cultured in 500 μL of RPMI 1640. Wells were divided into six groups with different final concentrations of PASODN: 0,1,2.5,5,10 and 20 μmol/L, respectively, the first one acted as a control. The medium was changed every other day. Ten days later, the cells in each well were stained with Giemsa and the colony-forming units were counted (consisting of more than 50 cells) under a phase contrast microscope. The optimal concentration of PASODN was screened according to the relative rate of inhibition of colony forming according to the formula: the relative rate of inhibition of colony-forming ( % ) = (1-numer of clone in experiment group/ number of clones in control group ) × 100%.
Logarithmic phase cells were digested, suspended and calculated, and then suspension was transferred to 6-well plates. Cell concentration was 400 cells/mL in each well. Wells were divided into: PASODN group, PMSODN group, and PBS group. The later two groups acted as controls. Twenty hours after the cells were adhered, RPMI 1640 was removed and wells were washed with 2.5 × PBS. Cells were incubated with 2 mL of RPMI 1640 culture medium containing 10 μmol/L PASODN, 10 μmol/L PMSODN and PBS (same volume). Medium was changed every other day. The incubation was terminated 10 d later. The colony-forming units were stained by Giemsa dye and counted. Colony-forming efficiency = (unit number /cell number) × 100%.
LS-174T cells (10 × 10 3 cells/mL in each well) were transferred to 24-well plates. Wells were divided into three groups and the cells were treated as mentioned above in 1 mL of RPMI1640 culture medium. Medium was changed every other day. The number of cells in each group was counted every day, and morphological changes were observed. The growth curve of the cells was obtained 13 d later.
PCR-ELISA was performed following the manufacturer’s instructions. All solutions were provided by the kit from Roche.
Briefly, the cells were digested, collected and centrifuged at 800 r/min for 10 min seven days after treatment with the reagents mentioned above. The supernatant was discarded. A total volume of 200 mL of the refrigerated lysis medium was added into a tube and placed on ice for 30 min. The cells were centrifuged at at 16000 g for 20 min at 4 °C. Then 100 mL of the supernatant was removed into a new tube. The telomere sequence was amplified in a 50 mL PCR system containing 25 mL of reaction solution, 2 mL of supernatant, 23 mL of triple-distilled water. PCR amplification was performed in the 9600 thermal cycler (PEPKIN ELMER): one cycle of primer extension at 25 °C for 30 min, 2 cycles of the inactivation at 94 °C for 5 min, 3-32 cycles of amplification at 94 °C for 30 s, at 50 °C for 30 s, at 72 for 90 s. The amplification products were stored at 4 °C.
The hybridization and ELISA were carried out as described below.
Denaturation solution and PCR products were incubated at room temperature for 10 min, 225 mL of hybridization solution was added and mixed thoroughly. Then 100 mL of the mixture was put in a 96-wells plate (provided by the kit), and the plate was vibrated (300 r/min) while it was incubated at 37 °C for 2 h. All the mixture was removed and the plate was rinsed 3 times. One hundred mL of anti-DIG peroxidase was added to each well, vibrated (300 r/min) and incubated at room temperature for 30 min. The solution was completely removed and each well was rinsed 5 times, 100 mL of substrate solution was added and incubated at room temperature for 15 min. Then the substrate solution was reserved, 100 mL stop solution was added into each well to terminate the discoloration development. The absorbance of the samples was measured ( △SA value) at 450 nm and at 690 nm within 30 min after termination of the reaction. If the value of △SA of the sample was higher than ‘0.2 △SA450 nm-△SA690nm’, then the telomerase in the sample was regarded as positive. The final value was expressed as mean ± SD, a statistical treatment was performed by using t test.
Cells in logarithmic phase were incubated with 10 μmol/L PASODN for 72 h and subsequently harvested by trypsinization, beated upon with PRMI 1640 culture containing serum, and centrifuged at 1000 r/min for 10 min. The supernatant was removed. The cells were fixed with 700 mL/L cold ethanol. FCM analysis of the DNA content was performed on a flow cytometer (FACS CaliburTM B.D USA)
The data were expressed as mean±SD. Analysis of data was performed using student’s t test. P < 0.01 was considered as statistically significant.
Although colony formation in LS-174T cells was inhibited by PASODN at the lower concentrations (1, 2.5, 5 μmol/L) in a dose-dependent manner, 10 μmol/L seemed the best concentration and the relative rate of inhibition was 82.8% (slightly lower than that in 20 μmol/L group) (Table 1).
| Clones | Concentration of PASODN (μmol/L) | |||||
| 0 | 1 | 2.5 | 5 | 10 | 20 | |
| Mean | 64.7 | 51.5 | 41.0 | 24.7 | 11.1 | 8.4 |
| Inhibitive rate (%) | 0 | 20.4 | 36.6 | 61.8 | 82.8 | 87.0 |
The colony-forming efficiency of cells treated with PASODN was 10.3%, which was significantly lower than that in PMSODN group (P < 0.01, t = 3.174) and PBS group (P < 0.01, t = 3.263). No significant difference was observed between PMSODN group and PBS group (Table 2).
| PASODN(10 μmol/L) | PMSODN(10 μmol/L) | PBS(same volume) | |
| Mean±SD | 41.2 ± 5.5 | 196.3 ± 13.5 | 202.8 ± 8.5 |
| Efficiency (%) | 10.3 | 49.1 | 50.7 |
| vs PASODN | P < 0.01 | P < 0.01 |
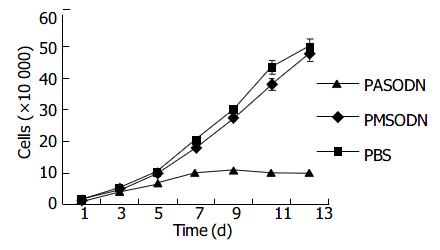
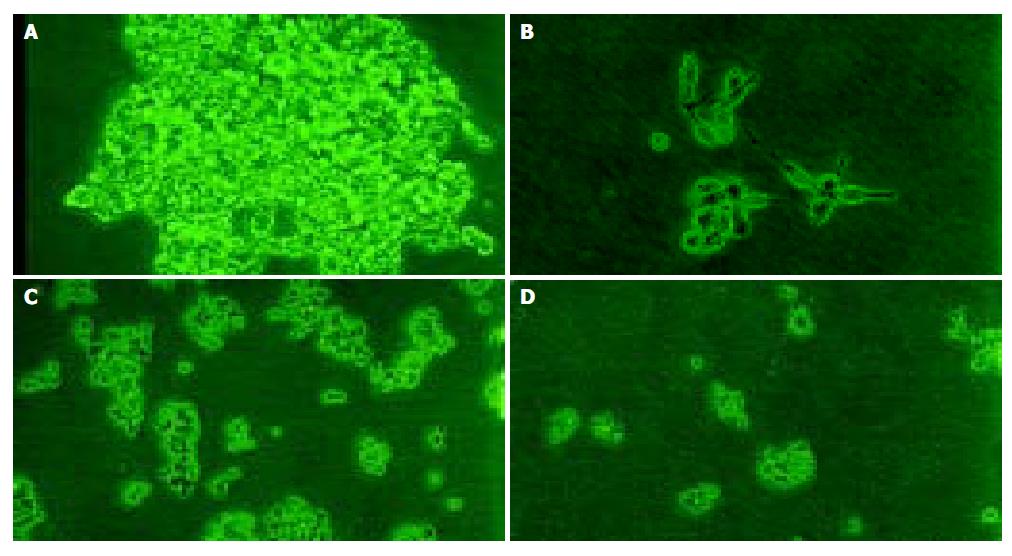
No significant effect was observed during the initial 5 d, but a time-dependent effect was observed during the subsequent days (Table 3 and Figure 1). The growth inhibition was observed obviously from the 7th d, which reached the peak from the 11th to 13th d. But PMSODN had no evident effect on cell growth. Under microscopy, obvious morphological changes were observed in the cells of experiment group as follow. Cells turned round, cell membrances shrank, and nuclei were concentrated at the karyothecae. The number of cells was very small and cells did not compact with each other. On the other hand, cells in other groups grew bloomy and tightly (Figure 2).
| Group | SA | P |
| PASODN | 0.18 ± 0.12 | |
| PMSODN | 1.89 ± 0.24 | < 0.01 |
| PBS | 1.98 ± 0.25 | < 0.01 |
Telomerase activity in PASODN group had no change. The △SA value of telomerase activity in PASODN group was significantly different from that in the other two control groups (P < 0.01, t = 3.317 and 3.241, t0.01(20)= 2.845) (Table 3).
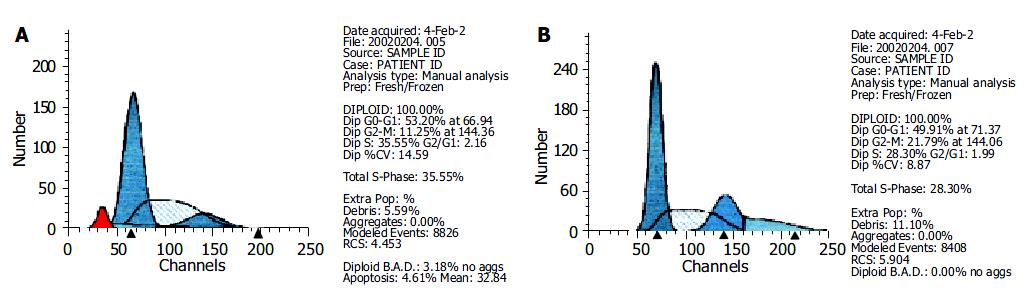
FCM analysis showed that the proportion of cells in G0/G1 and S phases increased appreciably 72 h after 10 μmol/L PASODN treatment, but no difference was observed between experiment and control groups (Table 4). A hypo diploid apex representing the apoptotic cells, was found before the apex of G0/G1 in the experiment group (Figure 3A), but not in control group (Figure 3B).
| Groups | G0/G1 | G2/M | S |
| Control | 49.91 | 21.79 | 28.30 |
| PASODN | 53.20 | 11.25 | 35.55 |
With the development of living conditions, the incidence of colorectal cancer has been going up year by year. Colorectal cancer is not sensitive to traditional chemotherapy[9]. So, it seems important to exploit potent and nontoxic drugs in this field. Telomerase activity has been reported to express in cancer but not in most normal somatic cells, suggesting that telomerase might be an important target for chemotherapy. Telomerase consists of an RNA component (hTR) that binds to telomere and a protein component (hTERT) that acts as a reverse transcriptase. In our opinion, targeting the template region of hTR with anti-sense reagents (such as oligonucleotides) may offer more advantages than targeting hTERT gene. It has been reported that the expression of telomerase activity depends on Watson-Crick base pair between hTR gene and telomere[10]. This accessibility and indispensability make hTR an ideal and convincible target for oligonucleotides.
PASODN exerted its inhibitory effect on LS-174T cells both at the cellular and molecular levels, whereas no such effects were observed in the control groups. Moreover, the inhibitory effect was time and dose-dependent. The inhibitory effect of PASODN on the cells had a certain latent phase. The growth curve showed that the inhibitory effect of PASODN on the growth of LS-174T cells was obvious on the seventh day, and reached the greatest value from the 11th to 13th d. Besides, the growth curve combined with the result of telomerase activity showed that after telomerase activity turned negative (on the 7th d), the cells could still continue to cleavage a certain number of times, and the phenomenon might be the “retarded effect” of telomerase inhibitor treatment.
In conclusion, specific oligonucleotide sequences can inhibit telomerase activity and lead to apoptotic cell death. The intrinsic existence of hTR in malignant tumors makes telomerase an ideal target for anti-sense reagent attack. But, we must pay attention to the fact that hTR also exists in normal somatic cells[11,12]. How to improve the tumor-affinity to oligonucleotids is still a problem. The lag phase[13] indicated that anti-telomerae inhibitors were unlikely to produce acute anti-proliferative effects. However, with researches going on in the field of telomerase and the advanced experiences of oligonucleotides in other molecular fields[14-16], the trial in animals and humans will be taken in near future.
Edited by Wang XL and Kumar M Proofread by Xu FM
| 1. | Morin GB. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989;59:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1068] [Cited by in RCA: 1122] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 2. | Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1302] [Cited by in RCA: 1318] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 3. | Kim NW. Clinical implications of telomerase in cancer. Eur J Cancer. 1997;33:781-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 124] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Wang XS, Wang K, Zhang QF, Chen F, Zhang DZ, Fu SB. Clini-cal significance of determination of telomerase activity in gastric cancer. Zhongguo Zhongliu Linchuang Zazhi. 2001;28:450-453. |
| 5. | Shong SH, Dong XS, Wang XS, Gao DQ, Fu SB, Li Y. Study on telomerase activity in human colorectal carcinoma and meta-static lymph nodes. Haerbin Yike Daxue Xuebao. 2002;36:29-31. |
| 6. | Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5156] [Cited by in RCA: 5233] [Article Influence: 168.8] [Reference Citation Analysis (0)] |
| 7. | Zhang FX, Zhang XY, Fan DM, Deng ZY, Yan Y, Wu HP, Fan JJ. Antisense telomerase RNA induced human gastric cancer cell apoptosis. World J Gastroenterol. 2000;6:430-432. [PubMed] |
| 8. | Kyo S, Inoue M. Complex regulatory mechanisms of telomerase activity in normal and cancer cells: how can we apply them for cancer therapy? Oncogene. 2002;21:688-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Bearss DJ, Subler MA, Hundley JE, Troyer DA, Salinas RA, Windle JJ. Genetic determinants of response to chemotherapy in transgenic mouse mammary and salivary tumors. Oncogene. 2000;19:1114-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Egholm M, Buchardt O, Christensen L, Behrens C, Freier SM, Driver DA, Berg RH, Kim SK, Norden B, Nielsen PE. PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen-bonding rules. Nature. 1993;365:566-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1599] [Cited by in RCA: 1557] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 11. | Hiyama E, Hiyama K. Clinical utility of telomerase in cancer. Oncogene. 2002;21:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 140] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1680] [Cited by in RCA: 1660] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 13. | Corey DR. Telomerase inhibition, oligonucleotides, and clinical trials. Oncogene. 2002;21:631-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Crooke ST. Potential roles of antisense technology in cancer chemotherapy. Oncogene. 2000;19:6651-6659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Lebedeva I, Stein CA. Antisense oligonucleotides: promise and reality. Annu Rev Pharmacol Toxicol. 2001;41:403-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 172] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Grillone LR, Lanz R. Fomivirsen. Drugs Today (Barc). 2001;37:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |