Published online Dec 1, 2004. doi: 10.3748/wjg.v10.i23.3428
Revised: April 19, 2004
Accepted: May 9, 2004
Published online: December 1, 2004
AIM: To determine the effect of metastatic hepatoma cells on lymphangioma-derived endothelium, and to establish in vitro model systems for assessing metastasis-related response of lymphatic endothelium.
METHODS: Benign lymphangioma, induced by intraperitoneal injection of the incomplete Freund’s adjuvant in BALB/c mice, was embedded in fibrin gel or digested and then cultured in the conditioned medium derived from hepatoma H22. Light and electron microscopy, and the transwell migration assay were used to determine the effect of H22 on tissue or cell culture. Expressions of Flt-4, c-Fos, proliferating cell nuclear antigen (PCNA), and inducible nitric oxide synthase (iNOS) in cultured cells, and content of nitric oxide in culture medium were also examined.
RESULTS: The embedded lymphangioma pieces gave rise to array of capillaries, while separated cells from lymphangioma grew to a cobblestone-like monolayer. H22 activated growth and migration of the capillaries and cells, induced expressions of Flt-4, c-Fos, PCNA and iNOS in cultured cells, and significantly increased the content of NO in the culture medium.
CONCLUSION: Lymphangioma-derived cells keep the differentiated phenotypes of lymphatic endothelium, and the models established in this study are feasible for in vitro study of metastasis-related response of lymphatic endothelium.
-
Citation: Yu H, Zhou HZ, Wang CM, Gu XM, Pan BR. Effect of hepatoma H22 on lymphatic endothelium
in vitro . World J Gastroenterol 2004; 10(23): 3428-3432 - URL: https://www.wjgnet.com/1007-9327/full/v10/i23/3428.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i23.3428
Metastasis of most cancers occurs primarily through the lymphatic system, and is responsible for the majority of cancer deaths. But tumor-associated lymphatic system has been overshadowed by the greater emphasis placed on the blood vascular system[1]. This scenario is changing rapidly after the identification of lymphangiogenic vascular endothelial growth factor C[2]. The traditional view that lymphatic capillaries are passive participants in metastasis is currently being challenged, and recent studies indicate the importance of lymphatic vessel activation in tumor dissemination[3-5].
Better understanding of the lymphatic endothelial properties and their alteration in cancer may develop a new way to therapeutic intervention[6,7]. In vitro experiments have been proven to be valuable, expeditious and easy of quantification in providing initial information on angiogenesis, a potentially important oncotherapy target[8]. However such in vitro models have not been well established for revealing metastasis-related response of lymphatic endothelium. Therefore, the aim of our study was to determine the effect of metastatic hepatoma cells on lymphangioma-derived endothelium, and to establish in vitro model systems for assessing metastasis-related response of lymphatic endothelium.
BALB/c mice of either gender, 2 mo old and weighing 22-25 g, were provided by Laboratory Animal Research Center, Fourth Military Medical University (FMMU, Xi’an, China). A mouse ascitic hepatoma cell line, H22, was obtained from Institute of Digestive Diseases, FMMU. Reagents for in vitro culture of lymphatic endothelium included incomplete Freund’s adjuvant, Hanks’ balanced salt solution (HBSS), M199 medium and fetal calf serum (FCS), which were purchased from Gibco Company (Carlsbad, California, USA), bovine fibrinogen, thrombin, gelatin, endothelial cell growth supplement (ECGS), heparin, collagenases I and II, which were products of Sigma Company (Saint Louis, Missouri, USA). The sABC and sABC-AP kits for immunohistochemical staining were purchased from Boster Company (Wuhan, China). Polyclonal anti-Flt-4 antibodies, monoclonal anti-iNOS antibody, anti-c-Fos antibody, and anti-PCNA antibody were products of Santa Cruz Company (Santa Cruz, California, USA). Nitric oxide (NO) detection kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Millicell culture plate inserts (12.0 μm) for transwell cell migration test were purchased from Millipore Corporation (Billerica, Massachusetts, USA).
Hepatoma H22 cells were inoculated into abdominal cavity of 5 BALB/c mice for passage. To verify the capability of spontaneous lymphatic metastasis of H22, the generated carcinomatous ascites was transplanted into hindlimb claw pad of 10 healthy BALB/c mice and the lymph nodes in groin were harvested 10 d later for pathological examination. The conditioned medium of H22 (H22 CM) was prepared as a mixture of sterile ultra-filtrate of H22 ascites and M199 medium at the ratio of 1:5, supplemented with 150 mL/L FCS. The control medium was M199 medium supplemented with 150 mL/L FCS.
Twenty healthy animals of BALB/c strain were intraperitoneally injected twice, with a 15-d interval, with 200 µL of the emulsified (1:1 with HBSS) incomplete Freund’s adjuvant and killed one month later. Multicentric and clearly delimited white neoplasm on the abdominal surface of the diaphragm, varying in size from 3 to 15 mm2, was collected for the following experiments. Histopathological examination of the tumors in our preparative experiment and other previous studies[9] confirmed that this neoplasm was benign lymphangioma.
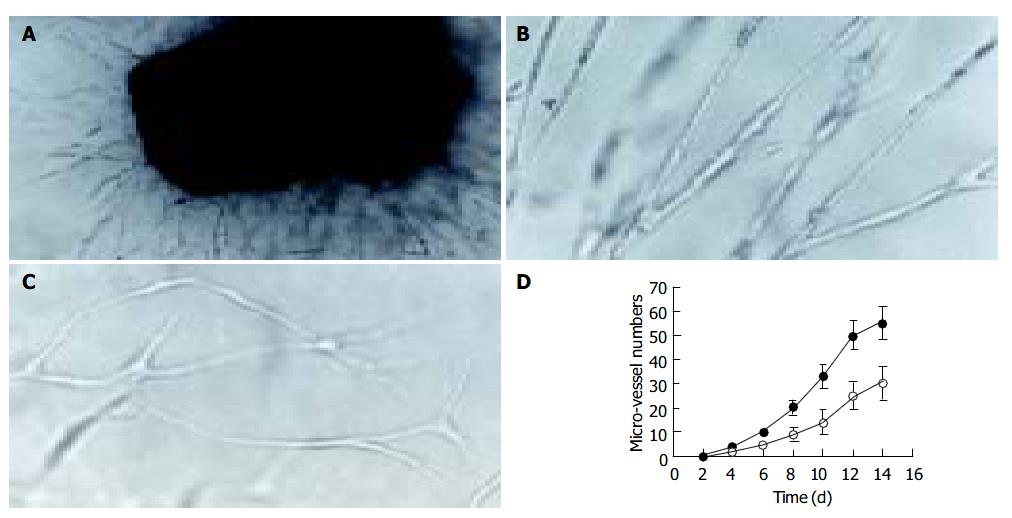
The lymphangioma masses were washed by HBSS, and cut into 1-mm2 pieces. HBSS containing 3 g/L bovine fibrinogen was added into 48-well culture plates (0.5 mL/well), and fibrin gel clotting was induced by addition of 10 µL 50 kU/L thrombin. A piece of lymphangioma was then placed on gel surface, and additional 0.5 mL fibrinogen solution and 10 µL thrombin were added to embed the tissue. H22 CM was then added into 24 wells (as an experimental group), and the control medium into another 24 wells (as a control group). The gels were incubated, with culture medium changed every other day, at 37 °C with 50 mL/L CO2 in air, and examined daily under an inverted microscope. To perform a quantitative analysis, images were taken every second day under same conditions (in brightness, contrast, and magnification), and measured by a computer-assisted image analysis system of Quantimet 570. For electron microscopy, six gels from each group were fixed two weeks later with 10 g/L glutaraldehyde in 0.1 mol/L sodium phosphate buffer, post-fixed with 10 g/L OsO4 in s-collidine buffer, dehydrated in graded ethanol, and embedded in Epon. Ultrathin sections were cut and stained with lead citrate and examined with a JEM-200EX transmission electron microscope.
Lymphangioma masses were mechanically broken, and washed in M199 medium, supplemented with 100 kU/L penicillin. The tissues were then incubated for 30 min at 37 °C in HBSS containing 0.5 g/L collagenases (1:1 mixture of type I and II), and washed with a medium containing 150 mL/L FCS to stop digestion. Cells were centrifuged at 700 r/min, resuspended in M199 medium supplemented with 150 mL/L FCS, and equally seeded on 10 g/L gelatin-coated cover slips in a 24-well plate. To allow cell attachment, the plates were put still for one hour in an incubator at 37 °C with 50 mL/L CO2 in air. Then the wells were gently washed to remove floating cells, and refilled with 2 mL H22 CM (24 wells) or 2 mL control medium (24 wells). Attached cells were cultured routinely, and examined daily under a phase-contrast microscope.
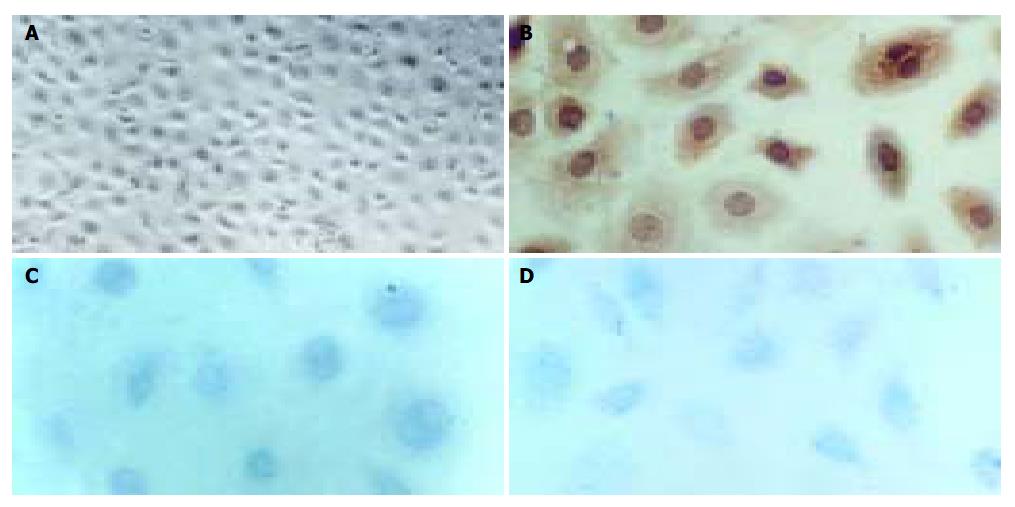
Six days after primary cell seeding, the cover slips were fixed with 40 g/L formaldehyde in PBS for 20 min, with 2 g/L Triton X-100 in PBS, and incubated with 200 mL/L normal goat serum. Polyclonal anti-Flt-4 antibodies, monoclonal anti-iNOS antibody, anti-PCNA antibody and anti-c-Fos antibody were used as primary antibodies, and biotin-labeled goat anti-rabbit IgG antibodies as second antibodies. Streptavidin and biotinylated horseradish peroxidase complex (sABC) and DAB were used for anti-Flt-4 and anti-iNOS antibodies, and sABC-AP (alkaline phosphatase) and BCIP/NBT for anti-PCNA and anti-c-Fos antibodies.
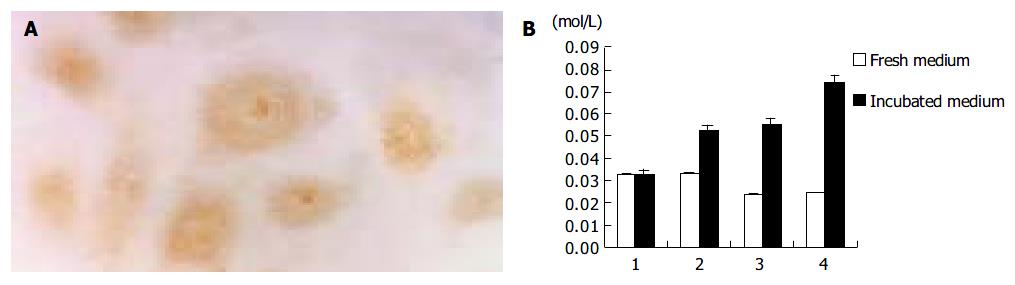
The content of NO in fresh H22 CM and control medium was indicated by determining the total concentration of nitrite/nitrate by using the colorimetric method, according to the instructions provided with the NO detection kit. After primary cells were equally seeded and cultured with 2 mL H22 CM or control M199 medium for 48 h, the levels of NO in the two media were detected again. For an empty control, the two media were added into an empty plate, which was then incubated at 37 °C for 48 h before NO detection.
Primary cells from lymphangioma were cultured to a confluent monolayer with a complete endothelium growth medium (M199 supplemented with 100 mg/L heparin, 30 mg/L ECGS and 150 mL/L FCS), subjected to serum starvation for 4 h by switching the culture medium to M199 medium without growth factors or serum, trypsinized and suspended (1 × 10 8 cells/L) in serum-free M199 medium. Transwell migration assays were performed in Millicell inserts, with 1 g/L gelatin-coated 12 µm filter membrane, in a 24-well culture plate. H22 CM and the control medium were added, respectively, into 12 wells of the plate, and 0.5 mL of suspended cells was placed in chambers of the inserts. After 4-h incubation at 37 °C, the medium in the insert chamber was aspirated, and cells on the upper surface of the filter membrane were removed with a cotton swab. Cells on the lower surface were fixed, stained with hematoxylin and counted in 5 high-power fields per chamber under light microscope.
Spontaneous lymphatic metastasis of transplant H22 tumor was found in all 10 animals as demonstrated by pathological examination of the groin lymph nodes (Figure 1). H22 CM could be easily prepared, and stored for the entire experiments to ensure the continuity of the different parts of the assay. The induction of lymphangioma was also confirmed to be a simple and well duplicable procedure, and could provide adequate original tissues for the following experiments.
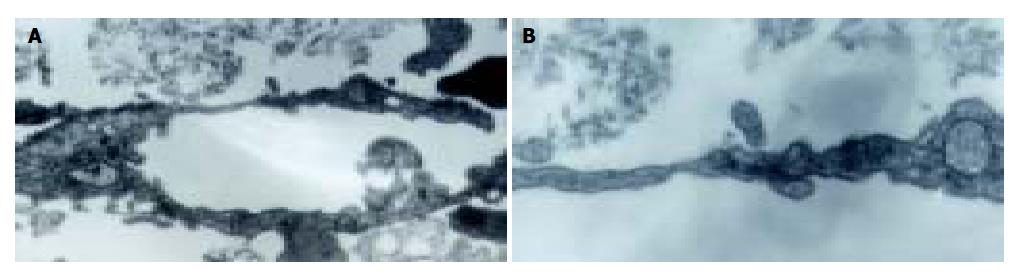
Lymphangioma embedded in the fibrin gels gave rise to capillaries when cultured 3-4 d in both H22 CM and control M199 media. Neo-branches of the micro-vessels with obvious lumina were generated every day. Under the effect of H22 CM, rather straight capillaries stretching out to the fringe of the gel were generated, and the number of branching in micro-vessels increased more rapidly (Figure 2). Transmission electron microscopy revealed that the ultrastructure of the capillaries was plasmalemma invagination of a single cell, or formed by cytoplasmic flaps joined by overlapping contacts and specialized tight junction complexes (Figure 3). End-to-end intercellular contacts were rare. No typical Weibel-Palade body was distinguished in the cytoplasm.
Primary cell culture was successfully established on gelatin-coated cover slips. When cultivated in H22 CM, the cells proliferated, albeit at a low rate with a doubling time of about 36-60 h, and reached sub-confluence in 6 d. At this stage, the lymphangioma cells showed appearance like cobblestone, the classical morphology of endothelial cells. In contrast, when cultured in the control M199 medium, the cells showed no obvious proliferation after attachment. Correspondingly, immunocytochemical staining for Flt-4, PCNA, and c-Fos were positive in cells cultured in H22 CM (Figure 4), but negative in cells cultured in the control medium.
Immunostaining showed the expression of iNOS in lymphangioma cells after culture in H22 CM, and significantly increased content of NO in the culture medium (Figure 5). In contrast, cells cultured in the control M199 medium did not express iNOS, and the level of NO was rather low in the control medium.
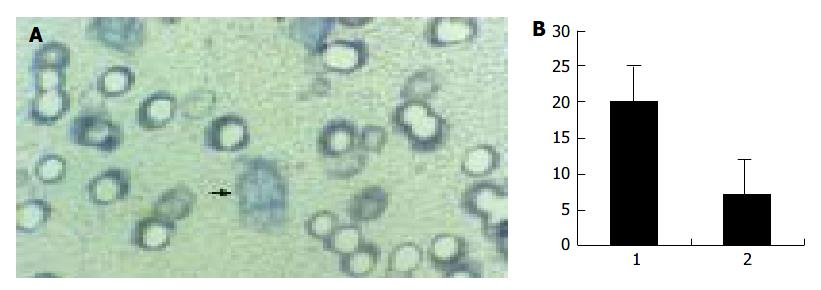
When cultured in the complete medium, the lymphangioma cells grew to a confluent monolayer in 8-10 d, and showed specialized cobblestone morphology, which further confirmed the nature of the cells. In transwell migration assay, the number of the cells on the lower surface of the filter membrane in H22 CM was significantly greater than in control medium (Figure 6).
The ascitic hepatoma H22 is a cell line with high potential of spontaneous lymphatic metastasis[10], which was also verified in our experiments, and has been generally used in various experimental studies in oncology[11-14]. It is proven to be a convenient and feasible tumor model for in vitro assessment on metastasis-related response of lymphatic endothelium.
The difficulty in isolation of lymphatic endothelium, especially from the animals commonly used in oncological experiments such as mice and rats, has limited studies on its reactions in tumorous environments. We used induced lymphangioma to solve this problem. When cultured in the fibrin gel, lymphangioma sprouts capillaries with ultrastructure similar to that of lymphatic vessels[4,15]. Moreover, gelatin-anchorage dependent primary cell culture, and cobblestone morphology of confluent or subconfluent monolayer, are also specialized phenotype of lymphatic endothelium[16,17]. Finally, positive expression of Flt-4 (also known as VEGFR-3), a marker of lymphatic endothelium in proliferation[18,19], confirmed the nature of the endothelium. These findings prove that lymphangioma is a proper original tissue for in vitro assay on lymphatic endothelium or lymphangiogenesis.
Despite the possible lymphangiogenic effect of fibrin[20], H22 CM induced a faster formation of capillary network from lymphangioma in fibrin gels. Moreover, in primary cell culture, H22 CM showed similar capability to the complete growth medium in activating proliferation of lymphatic endothelial cells. Immunostaining for the common proliferating nuclear marker, PCNA[21], and early-response gene signaling factor, c-Fos[22], further confirmed the microscopic findings. Our results accord with the opinion on lymphatic vessel activation in cancer.
Endogenous NO is one of the key factors in the control of dilation of the lymphatic micro-vessels[23,24]. The expression of iNOS in cancers is related to lymph node metastasis[25,26]. iNOS is also evenly distributed throughout the cytoplasm of lymphatic endothelial cells[27-29]. Our study demonstrates sustained expression of iNOS in cultured lymphatic endothelium and marked increase of NO levels in the culture medium under the effect of H22 CM, suggesting that dilation of lymphatics may be important for cancer invasion and metastasis.
There have been rare assays on lymphatic endothelial cell migration, which is an important event that initiates and coordinates lymphangiogenesis[30]. Results from our study suggest that some soluble factors in H22 CM activate migration of lymphatic endothelial cells and tropistic lymphangiogenesis. The coadjutant models established in our experiment are useful for further studies on migration inhibitors.
To our knowledge, this study is the first of its kind that bridges the gap between in vivo and in vitro studies on lymphatic endothelium and lymphangiogenesis. Both the culture medium and gel matrix in the experiment can be manipulated pharmacologically, which allows us to evaluate the activity of either soluble or solid-phase lymphangiogenesis inhibitors. Moreover, this spontaneous lymphatic metastasis model of transplant H22 tumor will be helpful to determine whether inhibition of lymphangiogenesis is a realistic therapeutic strategy for inhibiting tumor dissemination and metastasis.
Co-first-authors: Hua Yu and Hong-Zhi Zhou
Edited by Xia HHX Proofread by Zhu LH and Xu FM
| 1. | Pepper MS. Lymphangiogenesis and tumor metastasis: myth or reality? Clin Cancer Res. 2001;7:462-468. [PubMed] |
| 2. | Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290-298. [PubMed] |
| 3. | Nathanson SD. Insights into the mechanisms of lymph node metastasis. Cancer. 2003;98:413-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 314] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | Pepper MS, Skobe M. Lymphatic endothelium: morphological, molecular and functional properties. J Cell Biol. 2003;163:209-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 136] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Cassella M, Skobe M. Lymphatic vessel activation in cancer. Ann N Y Acad Sci. 2002;979:120-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Stacker SA, Hughes RA, Achen MG. Molecular targeting of lymphatics for therapy. Curr Pharm Des. 2004;10:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Mou JH, Yan XC, Wang D, Wu XH, Li ZP, Xiang DB. Relation-ship between VEGF-C expression and lymph node metastasis and prognosis in large intestinal carcinoma. Shijie Huaren Xiaohua Zazhi. 2004;12:1061-1064. |
| 8. | Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: a critical overview. Clin Chem. 2003;49:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 500] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 9. | Mancardi S, Stanta G, Dusetti N, Bestagno M, Jussila L, Zweyer M, Lunazzi G, Dumont D, Alitalo K, Burrone OR. Lymphatic endothelial tumors induced by intraperitoneal injection of incomplete Freund's adjuvant. Exp Cell Res. 1999;246:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Jiang XP, Tang ZY, Liu KD, Zhou XD, Lin ZY, Ling MY, Wu XF. mRNA levels of nm23 in murine ascites hepatoma (H22) clones with different lymphatic metastatic potential. J Cancer Res Clin Oncol. 1996;122:55-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Zhang J, Zhang JK, Zhuo SH. A vaccine prepared by fusion of H22 cells with the spleen-drived dendritic cells. Shijie Huaren Xiaohua Zazhi. 2004;12:276-279. |
| 12. | Yuan SF, Wang L, Li KZ, Yan Z, Han W, Zhang YQ. Inhibitory effect of MUC1 gene immunization on H22 hepatocellular carcinoma growth. Shijie Huaren Xiaohua Zazhi. 2003;11:1322-1325. |
| 13. | Liang J, Sun JY, Xie YH, Li Y, Yan L, Wang SW. Effect of monoclonal antibody 3A5 coupled with Chinese medicine com-pound Andi in targeted treatment of hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 2003;11:404-407. |
| 14. | Li ZS, Zhu ZG, Yin HR, Chen SS, Lin YZ. Diversity of telomerase activity in human and murine tumor cells transfected with cytokine genes. Shijie Huaren Xiaohua Zazhi. 1999;7:194-196. |
| 15. | Azzali G. The lymphatic vessels and the so-called "lymphatic stomata" of the diaphragm: a morphologic ultrastructural and three-dimensional study. Microvasc Res. 1999;57:30-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Mizuno R, Yokoyama Y, Ono N, Ikomi F, Ohhashi T. Establishment of rat lymphatic endothelial cell line. Microcirculation. 2003;10:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Leak LV, Jones M. Lymphatic endothelium isolation, characterization and long-term culture. Anat Rec. 1993;236:641-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566-3570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1026] [Cited by in RCA: 1044] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 19. | Karkkainen MJ, Mäkinen T, Alitalo K. Lymphatic endothelium: a new frontier of metastasis research. Nat Cell Biol. 2002;4:E2-E5. [PubMed] |
| 20. | Palumbo JS, Potter JM, Kaplan LS, Talmage K, Jackson DG, Degen JL. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res. 2002;62:6966-6972. [PubMed] |
| 21. | Enholm B, Karpanen T, Jeltsch M, Kubo H, Stenback F, Prevo R, Jackson DG, Yla-Herttuala S, Alitalo K. Adenoviral expression of vascular endothelial growth factor-C induces lymphangiogenesis in the skin. Circ Res. 2001;88:623-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 163] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Deed R, Rooney P, Kumar P, Norton JD, Smith J, Freemont AJ, Kumar S. Early-response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non-angiogenic, high-molecular-weight hyaluronan. Int J Cancer. 1997;71:251-256. [PubMed] |
| 23. | Nakaya K, Mizuno R, Ohhashi T. B16-BL6 melanoma cells release inhibitory factor(s) of active pump activity in isolated lymph vessels. Am J Physiol Cell Physiol. 2001;281:C1812-C1818. [PubMed] |
| 24. | Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am J Physiol. 1998;274:R790-R796. [PubMed] |
| 25. | Niu XJ, Wang ZR, Wu SL, Geng ZM, Zhang YF, Qing XL. Relationship between inducible nitric oxide synthase expression and angiogenesis in primary gallbladder carcinoma tissue. World J Gastroenterol. 2004;10:725-728. [PubMed] |
| 26. | Song ZJ, Gong P, Wu YE. Relationship between the expression of iNOS,VEGF,tumor angiogenesis and gastric cancer. World J Gastroenterol. 2002;8:591-595. [PubMed] |
| 27. | Ji RC, Kato S. Histochemical analysis of lymphatic endothelial cells in lymphostasis. Microsc Res Tech. 2001;55:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Marchetti C, Casasco A, Di Nucci A, Reguzzoni M, Rosso S, Piovella F, Calligaro A, Polak JM. Endothelin and nitric oxide synthase in lymphatic endothelial cells: immunolocalization in vivo and in vitro. Anat Rec. 1997;248:490-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Marchetti C, Casasco A, Di Nucci A, Cornaglia AI, Reguzzoni M, Rosso S, Piovella F, Calligaro A, Polak JM. Immunolocalization of endothelin and nitric oxide-synthase in lymphatic vessels and cultured lymphatic endothelial cells. Microvasc Res. 1997;53:282-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Boardman KC, Swartz MA. Interstitial flow as a guide for lymphangiogenesis. Circ Res. 2003;92:801-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 231] [Article Influence: 10.5] [Reference Citation Analysis (0)] |