Published online Dec 1, 2004. doi: 10.3748/wjg.v10.i23.3419
Revised: March 26, 2004
Accepted: April 13, 2004
Published online: December 1, 2004
AIM: Peroxisome proliferator-activated receptor γ (PPARγ ) is known to regulate growth arrest and terminal differentiation of adipocytes and is used clinically as a new class of antidiabetic drugs. Recently, several studies have reported that treatment of cancer cells with PPARγ ligands could induce cell differentiation and apoptosis, suggesting a potential application as chemopreventive agents against carcinogenesis. In the present study, 3 different kinds of PPARγ ligands were subjected to the experiments to confirm their suppressive effects on liver carcinogenesis.
METHODS: Three PPARγ ligands, pioglitazone (Pio) (200 ppm), rosiglitazone (Rosi) (200 ppm), and troglitazone (Tro) (1000 ppm) were investigated on the induction of the placental form of rat glutathione S-transferase (rGST P) positive foci, a precancerous lesion of the liver,and liver cancer formation using a diethylnitrosamine-induced liver cancer model in Wistar rats, and dose dependency of a PPARγ ligand was also examined.
RESULTS: PPARγ ligands reduced the formation of rGST P-positive foci by diethylnitrosamine and induction of liver cancers was also markedly suppressed by a continuous feeding of Pio at 200 ppm.
CONCLUSION: PPARγ ligands are potential chemopreventive agents for liver carcinogenesis.
- Citation: Guo YT, Leng XS, Li T, Zhao JM, Lin XH. Peroxisome proliferator-activated receptor γ ligands suppress liver carcinogenesis induced by diethylnitrosamine in rats. World J Gastroenterol 2004; 10(23): 3419-3423
- URL: https://www.wjgnet.com/1007-9327/full/v10/i23/3419.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i23.3419
Carcinogenesis is a complex process that has been divided into three stages: initiation, promotion and progression[1]. These three stages of tumor formation have been characterized in many mammalian tissues, particularly in the liver[2,3]. According to the concept of multistage carcinogenesis, clones of cells arise with increasing autonomy from normal growth regulation at each stage of development, and from these selected populations of cells, neoplasms ultimately develop[4]. The placental form of rat glutathione S-transferase (rGST P) positive foci is thought to be preneoplastic lesions of the liver[5-12].
Peroxisome proliferators-activated receptor γ (PPARγ ), a nuclear hormone receptor, provides a strong link between lipid metabolism and regulation of gene transcription. PPARγ is known to regulate growth arrest and terminal differentiation of adipocytes, and a group of PPARγ activators are now widely prescribed and form a new class of antidiabetic drugs. Several specific ligands have been identified, such as the thiazolidinedione groups, including pioglitazone (Pio), rosiglitazone (Rosi), and troglitazone (Tro), 15-deoxy-prostaglandin J2, and certain polyunsaturated fatty acids[13]. PPARγ is expressed in various organs including adipose tissue, breast epithelium, small intestine, lungs, and liver[13]. Several studies have reported that treatment of cancer cells with PPARγ ligands could induce cell differentiation and apoptosis, suggesting a potential application as chemopreventive agents against carcinogenesis[14].
In present study, 3 different kinds of PPARγ ligands were subjected to the experiments to confirm their suppressive effects on liver carcinogenesis in the rat model. In experiment 1, we investigated the effects of 3 PPARγ ligands, Pio, Rosi, and Tro, on the development of rGST P-positive foci that were induced by diethylnitrosamine (DEN)[6]. Dose dependency of the PPARγ ligand was also examined. In experiment 2, a long-term carcinogenesis study of DEN was conducted[15]. Our findings indicate that PPARγ ligands are potential chemopreventive agents for liver carcinogenesis.
DEN was purchased from Beijing Chemical Reagents Company (Beijing, China). Three different PPARγ ligands, Pio, Rosi, and Tro, were kindly provided by GlaxoSmithKline (BN, United Kingdom). To prepare experimental diets, each PPARγ ligand was mixed with a powdered basal diet and stored at 4 °C until use. Fresh diets were provided to the rats once a week, and the dietary intake and body mass (bm) for each group of rats were measured every week. Concentrations of PPARγ in experimental diets were 200 and 1000 ppm for Pio and Tro, respectively. For Rosi, various concentrations in the diet, ranging from 0.88 to 500 ppm, were used to evaluate the dose-response effect of PPARγ ligands on the formation of rGST P-positive foci. Fifteen-week-old male Wistar rats were purchased from Peking University Health Science Center (Beijing, China) and housed in a ventilated, temperature-controlled room (23 ± 1 °C) with a 12-h light/dark cycle. After a period of 1-week acclimatization to the housing environment and the basal diet, groups of rats were fed either the basal diet or the experimental diet containing one of the PPARγ ligands, from sixteen weeks of age until termination.
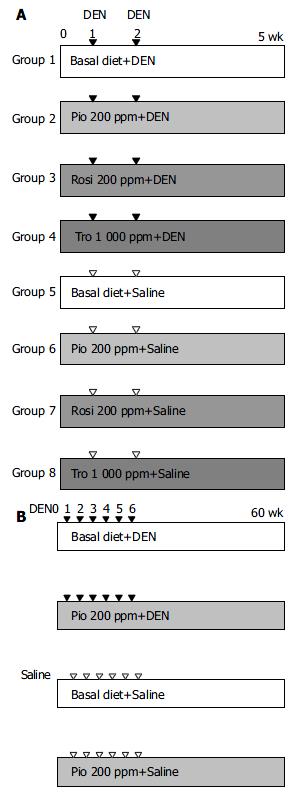
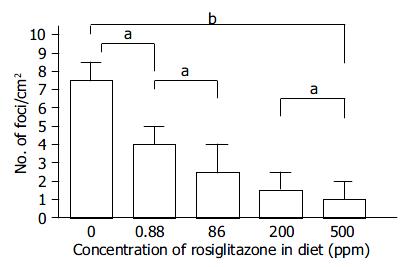
Rats were divided into 8 experimental groups, 4 DEN treated and 4 vehicle-treated. In DEN -treated cases (groups 1-4, Figure 1A), groups of 10 rats were fed diets without ligands (basal diet, group 1) or with a PPARγ ligand (experimental diet, groups 2-4), beginning 1 wk prior to the subcutaneous injection of DEN as described below and continued until the end of the experiment. Starting at sixteen weeks of age (experimental wk 1), rats in each group were administered DEN subcutaneously twice, at 1-week intervals at a dose of 20 mg/kg bm in saline. In the vehicle-treated groups (groups 5-8, Figure 1A), groups of 5 rats were fed the basal diet (group 5) or experimental diet (groups 6-8) and given subcutaneous injections of an equal volume of saline without DEN. Four weeks after the first administration of DEN or saline, all rats were killed at experimental week 5. In a separate experiment, dose-dependent effect of Rosi, within the range of 0.67-500 ppm in diet, on formation of rGST P-positive foci was also examined. The entire liver was removed, and fixed in 40 g/L neutralized formaldehyde overnight at 4 °C. Then the liver sections were embedded in paraffin. Immunohistochemical staining was performed on sections by the peroxidase-antiperoxidase method, using the antibody against the rGST P. Sections were counterstained with haematoxylin. The number of rGST P-positive foci (consisting of single to several GST P positive cells, indicating preneoplastic lesions) was expressed as per cm2 of liver sections[16].
As in Experiment 1, rats were fed either the experimental diet or the basal diet, starting 1 wk prior to the injection of DEN or vehicle. A total of 80 rats were divided into 4 experimental groups, 2 DEN treated and 2 vehicle treated. For the former, groups of 30 rats were fed diets without ligands (basal diet) or with PPARγ ligands (basal diet + pioglitazone 200 ppm), beginning 1 wk prior to the subcutaneous injection of DEN as described below and continued until the end of the experiment (Figure 1B). Starting at 16 wk of age (experimental week 1), rats in each group were administered DEN subcutaneously 6 times at 1-wk intervals at a dose of 20 mg/kg bm in saline. In the vehicle-treated groups, groups of 10 rats were fed the basal diet or experimental diet (basal diet + pioglitazone 200 ppm) and given subcutaneous injections of an equal volume of saline without DEN (Figure 1B). All surviving animals were killed 60 wk after the first injection of DEN. Livers were removed and fixed in 10% neutralized formalin as described above. The number, size, and location of liver tumors dectected macroscopically or by stereomicroscopic observation were determined. Diameters were measured in 3 dimensions, and tumor size was calculated by (height + width + length)/3.
Liver slices were embedded in paraffin blocks according to the standard procedures. In the case of liver tumors, lesions were resected, fixed in neutralized 40 g/L formaldehyde overnight at 4 °C, and embedded in paraffin blocks according to the standard procedures. Some of the tumors were bisected, and a half was frozen in liquid nitrogen and stocked at -80 °C until used for the frozen section preparation and RNA extraction. The other half was embedded in paraffin blocks as described above. Paraffin sections were prepared at 3.0-μm thickness, stained with H & E, and subjected to histologic analysis.
Paraffin-embedded sections were deparaffinized and subjected to immunohistochemical staining using rabbit alpha fetoprotein polyclonal antibody (Novus Biologicals, Inc.) and rabbit PPARγ polyclonal antibody (Santa Cruz Biotechnology, Inc.) following the manufacturer’s instructions. The primary alpha fetoprotein (AFP) antibody was diluted at 1:200 and the PPARγ antibody to 1:400. To confirm the specificity of PPARγ staining, addition of an excess amount of the N-terminal peptide of PPARγ , used for immunization, was also carried out.
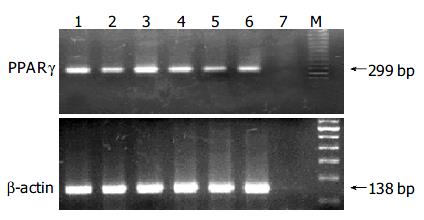
RNA extraction from liver tumors and surrounding normal liver tissues was carried out as follows. Serial sections were prepared at 7-μm thickness from frozen tissues, lesions were microdissected by scraping with a razor blade under microscopic observation, and RNA was extracted using TRI reagent (Sigma). Extracted RNA was transcribed to cDNA using Oligo(dT)12-18 primer and SuperScript II RT (Invitrogen, Carlsbad, CA) and subjected to semiquantitative reverse-transcriptase (RT)-PCR analysis to quantify messenger RNA (mRNA) expression levels of the PPARγ gene. PCR primers designed to amplify the 299-bp fragment of the PPARγ gene were 5’-AGGATTCATGAC-CAGGGAGTT-3’ (forward) and 5’-TCTGCCTGAGGTCT-GTCATCT-3’ (reverse). For the internal control, expression of the β -actin gene for each sample was also quantified as above by amplifying the 138-bp fragment using a primer set: 5’-AGACTTCGAGCAGGAGATGGC-3’ (forward) and 5’-AAGAAGGAAGGCTGGAAAAGA-3’ (reverse). PCR amplification was carried out at 94 °C for 30 s, at 59 °C for 30 s, and at 72 °C for 45 s using MasterMix kit (tw-biotech, China) under the reaction conditions recommended by the manufacturer, and PCR cycles were set at 30 and 25 cycles for PPARγ and β -actin, respectively. PCR products were run on 20 g/L agarose gels and visualized by ethidium bromide staining. In addition, the amount of PCR products was also quantified in real-time PCR using Taq RT-PCR kit (Ambion Inc.) and Smart cycler system (Cepheid, Sunny Vale, CA). Experiments were repeated at least twice, which gave similar results.
Statistical analysis for rGST P-positive foci and liver tumor multiplicity were conducted using the t-test (SPSS 10.0 for Windows). Other statistical analyses for liver tumor incidences and the distribution of tumor sizes were performed using the χ2-test (SPSS 10.0 for Windows). Differences were considered significant when P values were < 0.05.
One animal in the DEN + 200-ppm Rosi-treated group and 4 in the DEN + 1000-ppm Tro-treated group died within a few days after the injection of DEN, which were not included in the effective number of rats. Based on the diet consumption, the daily intake of PPARγ ligands was estimated to be approximately 25 mg/kg bm (200 ppm in diet) for Pio and Rosi and 125 mg/kg bm (1000 ppm in diet) for Tro. The mean body weight of rats given DEN was slightly decreased but stayed within 10% of the control value (data not shown). Administration of PPARγ ligands alone did not significantly alter the body mass within the observation period of 5 wk (data not shown). After the administration of DEN, rats fed with the basal diet without a PPARγ ligand developed 7.5 ± 3.1 rGST P-positive foci per cm2 of liver sections by experimental wk 5. Treatment with 200-ppm Pio, 200-ppm Rosi, and 1000-ppm Tro significantly reduced that to 2.5 ± 1.2, 1.7 ± 1.1, and 5.6 ± 2.3, respectively (Table 1). This suppressive effect was observed in a dose-dependent manner within the range of 0.88 to 500 ppm of Rosi (Figure 2). Histologic analysis of a total of 40 liver sections of rGST P-positive foci (10 liver sections of rGST P-positive foci each from groups 1, 2, 3, and 4) revealed no significant histologic change. No rGST P-positive foci were induced by feeding experimental diet or basal diet alone without DEN administration.
| Experimental group | DEN | Diet | No. of rats | No. of rGST P-positive foci/cm2 of liver sections |
| Group 1 | + | Basal diet alone | 10 | 7.5 ± 3.1 |
| Group 2 | + | Basal diet + pioglitazone 200 ppm | 10 | 2.5 ± 1.2a |
| Group 3 | + | Basal diet + rosiglitazone 200 ppm | 9 | 1.7 ± 1.1a |
| Group 4 | + | Basal diet + troglitazone 1000 ppm | 6 | 5.6 ± 2.3a |
| Group 5 | - | Basal diet alone | 5 | 0 |
| Group 6 | - | Basal diet + pioglitazone 200 ppm | 5 | 0 |
| Group 7 | - | Basal diet + rosiglitazone 200 ppm | 5 | 0 |
| Group 8 | - | Basal diet + troglitazone 1000 ppm | 5 | 0 |
Five animals treated with DEN + 200 ppm Pio died before the termination without tumor development, which were not included in the effective number. With administration of DEN, body weight gradually dereased to approximately 90% of the vehicle-treated group by experimental wk 18, but the difference stayed within 10% of the control mass throughout the experimental period. Data for incidences, multiplicities (average numbers of tumors per animal), histologic features of liver tumors, and size distribution of tumors are summarized in Table 2. Administration of 200-ppm Pio almost halved the incidence of liver tumors, and the multiplicity was also significantly reduced by Pio treatment. As to the size, only 38% (3 of 8) of tumors observed in the Pio-treated group were more than 2 mm in diameter, and 38% (3 of 8) were less than 1 mm. In contrast, 76% (19 of 25) of those in rats without Pio treatment were more than 2 mm in diameter, and only 8% (2 of 25) were less than 1 mm (P < 0.05). All of the lesions developed in both Pio-treated and untreated groups were diagnosed as hepatocellular carcinoma. No liver tumors were observed in the rats fed with the basal diet alone or diet with 200-ppm Pio (data not shown).
Liver tumors in both Pio-treated and untreated rats demonstrated no appreciable histologic differences on H & E staining or AFP immunostaining. Furthermore, no variation was observed in PPARγ expression levels in tumors developed in Pio-treated and untreated rats. Addition of an excessive antigenic PPARγ N-terminal peptide used for immunization before incubation of the sections with the antibody blocked the staining completely (data not shown).
RNA samples from 2 liver tumors and 1 normal liver sample from both Pio-treated and untreated groups were subjected to semiquantitative RT-PCR analysis. No products were amplified without reverse transcriptase (data not shown). Liver tumors and normal tissue from both Pio-treated and untreated animals expressed PPARγ mRNA as shown in Figure 3, and the relative amounts, normalized to β -actin mRNA, were almost equivalent in liver tumors as compared with normal liver tissue in both groups. Real-time PCR analysis also gave similar results (data not shown).
In the present study of short- and long-term effects, clear evidence was obtained for preventive influence of the 3 different PPARγ ligands, Pio, Rosi, and Tro, on rat liver carcinogenesis induced by DEN. In the short-term experiment, all 3 significantly suppressed the formation of rGST P-positive foci, the effect of the former 2 was particularly strong. Because this suppression was proved to be dose dependent, we considered the inhibitory effect of PPARγ ligands on the formation of rGST P-positive foci to be due to pharmacologic actions rather than nonspecific toxicity. There were no significant differences in histopathologic features of rGST P-positive foci. In this context, the fact that the compounds reduced the formation of rGST P-positive foci without otherwise affecting their pathology is important. In the long-term carcinogenic experiment, the single PPARγ ligand investigated also significantly suppressed the induction of liver tumors.
Our results are in good agreement with those from Rumi et al[17], which demonstrated an inhibitory effect of PPARγ on cell growth through PPARγ activation. Our study has an advantage of the suppressive effect of rGST P-positive foci and liver tumors using 3 PPARγ ligands in the rat model, including the dose-dependent experiments. In addition, PPARγ could play roles in apoptosis, cell-cycle control through regulating p21waf/cipl[18]. Antiinflammatory activity of PPARγ could also be involved in its suppression of liver carcinogenesis[19]. However, the results of Hosokawa et al[20] and Kim et al[21], showing clofibrate, a peroxisome proliferator, enhanced liver carcinogenesis in their rat model in which GST-P-positive cells induced by DEN were changed to GST-P-negative cells on subsequent treatment with peroxisome proliferator, need to be taken into account. Now we have no reasonable explanation for the discrepancy.
In conclusion, PPARγ ligands have substantial suppressive effects on liver carcinogenesis without affecting the histologic features of preneoplastic lesions or tumors. Although the molecular mechanisms underlying the inhibitory influence still remain largely unsolved, PPARγ ligands may be potential chemopreventive agents against liver carcinogenesis.
Edited by Zhang JZ and Wang XL Proofread by Xu FM
| 1. | Dragan YP, Pitot HC. The role of the stages of initiation and promotion in phenotypic diversity during hepatocarcinogenesis in the rat. Carcinogenesis. 1992;13:739-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Farber E, Solt D, Cameron R, Laishes B, Ogawa K, Medline A. Newer insights into the pathogenesis of liver cancer. Am J Pathol. 1977;89:477-482. [PubMed] |
| 3. | Pitot HC, Barsness L, Goldsworthy T, Kitagawa T. Biochemical characterisation of stages of hepatocarcinogenesis after a single dose of diethylnitrosamine. Nature. 1978;271:456-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 241] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Pitot HC, Dragan Y, Sargent L, Xu YH. Biochemical markers associated with the stages of promotion and progression during hepatocarcinogenesis in the rat. Environ Health Perspect. 1991;93:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Satoh K, Kitahara A, Soma Y, Inaba Y, Hatayama I, Sato K. Purification, induction, and distribution of placental glutathione transferase: a new marker enzyme for preneoplastic cells in the rat chemical hepatocarcinogenesis. Proc Natl Acad Sci U S A. 1985;82:3964-3968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 309] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Ito N, Tatematsu M, Hasegawa R, Tsuda H. Medium-term bioassay system for detection of carcinogens and modifiers of hepatocarcinogenesis utilizing the GST-P positive liver cell focus as an endpoint marker. Toxicol Pathol. 1989;17:630-641. [PubMed] |
| 7. | Reddy TV, Daniel FB, Lin EL, Stober JA, Olson GR. Chloroform inhibits the development of diethylnitrosamine-initiated, phenobarbital-promoted gamma-glutamyltranspeptidase and placental form glutathione S-transferase-positive foci in rat liver. Carcinogenesis. 1992;13:1325-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Zhu HZ, Zhang XL, Chen YS. Expression of glutathione S-transferase placental mRNA in hepatic preneoplastic lesions in rats. World J Gastroenterol. 1998;4:38-40. [PubMed] |
| 9. | Denda A, Kitayama W, Konishi Y, Yan Y, Fukamachi Y, Miura M, Gotoh S, Ikemura K, Abe T, Higashi T. Genetic properties for the suppression of development of putative preneoplastic glutathione S-transferase placental form-positive foci in the liver of carcinogen-resistant DRH strain rats. Cancer Lett. 1999;140:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Sporn MB, Suh N. Chemoprevention of cancer. Carcinogenesis. 2000;21:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 296] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Nishikawa T, Wanibuchi H, Ogawa M, Kinoshita A, Morimura K, Hiroi T, Funae Y, Kishida H, Nakae D, Fukushima S. Promoting effects of monomethylarsonic acid, dimethylarsinic acid and trimethylarsine oxide on induction of rat liver preneoplastic glutathione S-transferase placental form positive foci: a possible reactive oxygen species mechanism. Int J Cancer. 2002;100:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Suzuki S, Asamoto M, Tsujimura K, Shirai T. Specific differences in gene expression profile revealed by cDNA microarray analysis of glutathione S-transferase placental form (GST-P) immunohistochemically positive rat liver foci and surrounding tissue. Carcinogenesis. 2004;25:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Lambe KG, Tugwood JD. A human peroxisome-proliferator-activated receptor-gamma is activated by inducers of adipogenesis, including thiazolidinedione drugs. Eur J Biochem. 1996;239:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 153] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Chang TH, Szabo E. Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor gamma in non-small cell lung cancer. Cancer Res. 2000;60:1129-1138. [PubMed] |
| 15. | Dragan YP, Hully J, Baker K, Crow R, Mass MJ, Pitot HC. Comparison of experimental and theoretical parameters of the Moolgavkar-Venzon-Knudson incidence function for the stages of initiation and promotion in rat hepatocarcinogenesis. Toxicology. 1995;102:161-175. [PubMed] |
| 16. | Carter JH, Richmond RE, Carter HW, Potter CL, Daniel FB, DeAngelo AB. Quantitative image cytometry of hepatocytes expressing gamma-glutamyl transpeptidase and glutathione S-transferase in diethylnitrosamine-initiated rats treated with phenobarbital and/or phthalate esters. J Histochem Cytochem. 1992;40:1105-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Rumi MA, Sato H, Ishihara S, Kawashima K, Hamamoto S, Kazumori H, Okuyama T, Fukuda R, Nagasue N, Kinoshita Y. Peroxisome proliferator-activated receptor gamma ligand-induced growth inhibition of human hepatocellular carcinoma. Br J Cancer. 2001;84:1640-1647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Koga H, Sakisaka S, Harada M, Takagi T, Hanada S, Taniguchi E, Kawaguchi T, Sasatomi K, Kimura R, Hashimoto O. Involvement of p21(WAF1/Cip1), p27(Kip1), and p18(INK4c) in troglitazone-induced cell-cycle arrest in human hepatoma cell lines. Hepatology. 2001;33:1087-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 867] [Cited by in RCA: 862] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 20. | Hosokawa S, Tatematsu M, Aoki T, Nakanowatari J, Igarashi T, Ito N. Modulation of diethylnitrosamine-initiated placental glutathione S-transferase positive preneoplastic and neoplastic lesions by clofibrate, a hepatic peroxisome proliferator. Carcinogenesis. 1989;10:2237-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Kim DJ, Lee KK, Hong JT. Differential effects of nongenotoxic and genotoxic carcinogens on the preneoplastic lesions in the rat liver. Arch Pharm Res. 1998;21:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |