INTRODUCTION
Hepatocellular carcinoma is one of the most common cancers worldwide, especially in Asia[1]. It frequently shows early invasion into blood vessels together with intrahepatic extensions and later extrahepatic metastasis[2]. A better understanding of the processes involved in the development of the metastasis might improve future prognosis by facilitating treatment strategies.
Tumor invasion is a complex biological process, during which tumor cells detach from the primary tumor and infiltrate the surrounding tissue. This process requires loss of cell contacts between tumor cells, active cell migration, adhesion to the extracellular matrix and proteolytic degradation of the extracellular matrix[3-5]. Rho, a member of the Ras family of small GTP-binding proteins, has a molecular weight of approximately 27 kDa and is located under the cell membrane, potentially functioning in signal transduction pathways[6]. Small GTPase Rho protein is known to work as a molecular switch for the regulation of signal transduction of intracellular events related to cell motility, cytoskeletal dynamics, and tumor progression[7,8]. In the present study, we hypothesized that Rho protein might be associated with the development of hepatocellular carcinoma.
Lysophosphatidic acid (LPA) is a product of phospholipid metabolism. Exogenous LPA binds to surface G protein-coupled receptors and its biological activities are mediated in part by the cytosolic small GTPase Rho[9-11]. The motility of tumor cells could be regulated by the target of Rho, Rho-associated coiled-coil forming protein kinase(p160ROCK), through reorganization of the actin of cytoskeleton[12]. A recently described specific inhibitor of p160ROCK, the (+)-(R)-trans-4-(l-Aminoethyl)-N-(4-pyridyl) cyclohexanecarboxamide dihydrochloride (Y-27632), was reported to inhibit Rho-mediated cell migration as well as smooth muscle contraction both in vivo and in vitro[13-15].
Given the recent interest in the participation of Rho in cell migration, we analyzed the role of Rho associated signal transduction pathways during the LPA-induced transmigration of human hepatocellular cells, and more importantly the activity of Rho regulated by LPA and inhibitor of p160ROCK.
MATERIALS AND METHODS
Cell lines and treatment
SMMC-7721 cell, originally isolated from a poorly differentiated hepatocellular carcinoma, was used in all experiments. SMMC-7721 cell was cultured in modified minimum essential medium (MEM) containing 2-fold concentrated amino acids and vitamins supplemented with 10% fetal calf serum (FCS) at 37 °C in a humidified atmosphere of 5% CO2 in air. The cells were used within 15-20 passages after the initiation of cultures. Before each experiment, cells were cultured under serum-free conditions (in medium containing 0.1% bovine serum albumin) for 24 h.
Western blot analysis
Cells were starved in serum-free culture medium for 24 h and subsequently treated with LPA at 37 °C for various times. The cells were washed twice with PBS and lysed in ice-cold lysis buffer (20 mM Tris, pH7.4, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM glycerolphosphate, 1 mM sodium orthovanadate, 1 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride). The extracts were centrifuged to remove cellular debris, and protein content of the supernatants was determined using the Bio-Rad protein assay reagent. Samples were resolved by SDS-polyacrylamide gel electrophoresis and transferred to Hydbond-P. The transferred samples were incubated with the anti-rho antibody indicated, and then incubated with HRP-conjugated IgG, and the immunoblotted proteins were visualized with ECL reagents.
Rho activation assay
A commercial pull-down assay (Rho activation assay kit, Upstate) was used to measure the effect of LPA and Y-27632 on Rho activity in SMMC-7721 cells. The cells were washed twice with modified MEM and incubated in fresh modified MEM without serum for 24 h, 25 μM LPA with or without Y-27632 (25 μmol/L) was added and the cell suspension was centrifuged at the indicated time after LPA addition, and then the cell pellet was lysed (lysis buffer, Upstate) and the activated Rho pull-down assay was performed according to the manufacturer’s protocol. A protein assay was performed prior to beginning pull-down assay to equalize the total protein concentration of each treatment group. Positive and negative controls were also performed according to the manufacturer’s protocol. Briefly, lysates of cells were preincubated with 100 μM nonhydrolyzable GTPγS (positive Rho activation) or 100 μM GDP (negative Rho activation) prior to undergoing precipitation by the Rhotekin GTP-Rho binding domain.
Morphological changes by Rho-kinase pathway
The cells were cultured in serum-free culture medium, and the dishes were incubated for an additional period of 24 hours. LPA (5 μmol/L) was then added to the dishes with or without different concentrations of Y-27632 (5, 25, 125 μmol/L) and cultured for 1 hour. The cells were examined using a phase-contrast microscope.
Cell motility assay
Random motility was determined by using the gold-colloid assay[16]. Gold colloid was layered onto glass coverslips and placed into 6-well plates. Cells were seeded onto the coverslips and allowed to adhere for 1 h at 37 °C in a CO2 incubator (12 500 cells/3 ml in serum-free medium). To stimulate the cells, the serum-free medium was replaced with 5% FBS containing LPA (5 μmol/L) with or without Y-27632 (25 μmol/L) and allowed to incubate for 24 h at 37 °C. The medium was aspirated and the cells were fixed with 2% gluteraldehyde. The coverslips were then mounted onto glass microscope slides and areas of clearing in the gold colloid corresponding to phagokinetic cell tracks were counted.
Cell invasion assay
Chemotactic directional migration was evaluated using a modified Boyden chamber[17,18]. In this invasion assay, tumor cells had to overcome a reconstituted basement membrane by a sequential process of proteolytic degradation of the substrate and active migration. Costar transwells (pore size 8 µm) were coated with matrigel, dried at 37 °C in an atmosphere of 5% CO2, and reconstituted with serum-free medium. Cells (3×104) were plated in the upper chamber in medium containing LPA with or without Y-27632 and allowed to migrate for 4.5 h. Nonmigrating cells were removed from the upper chamber with a cotton swab and migrating cells adherent to the underside of the filter were fixed and stained with Mayer’s hematoxylin solution and counted with an ocular micrometer. All experiments were performed in triplicate, and at least 10 fields/filter were counted. Data were expressed as relative migration (number of cells/field) and represented as -x±s of at least three independent experiments.
Statistical analysis
All data were expressed as -x±s. Statistical analysis was done by statistical package of SPSS for window release 9.0. Differences between the mean were tested with the Mann-Whitney test. P < 0.05 was considered statistically significant.
RESULTS
Increase of Rho protein levels as a result of LPA treatment
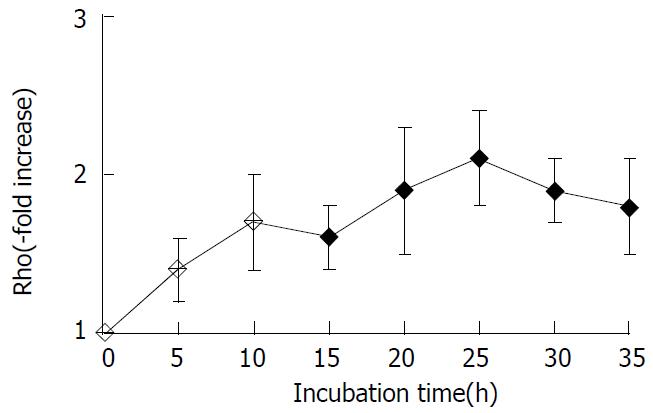
Using Western blot analysis with Rho polyclonal antibody, we detected the changes of the level of Rho family protein in SMMC-7721 cell extracts. The protein level of Rho rapidly increased within 5 h and reached the nearly maximal level at 25 h after the treatment with LPA, with more than 2-fold increase over the untreated control value (Figure 1).
Figure 1 Changes in levels of Rho family protein in SMMC-7721cells induced by LPA.
The levels of Rho protein were ana-lyzed by Western blot analysis. The data shown are expressed as -x±s from three different experiments.
Inhibition of LPA-induced Rho activation by Y-27632
We measured the intracellular levels of the GTP-bound active form of Rho by the pull-down assay system[19]. Activated GTP-Rho was precipitated by the Rho-binding domain of Rhotekin, a downstream Rho effector that specifically binds to only activated GTP-Rho[20]. The level of GTP-bound Rho was elevated transiently after the addition of LPA and reached the peak level at 30 min after LPA addition, whereas Y-27632 decreased the level of active Rho. Since the total amount of Rho in each lysate was almost constant, Y-27632 inhibited Rho activation. This result was controlled by equalizing the harvested protein levels from each treatment with an appropriate dilution of buffer determined by a protein concentration assay prior to beginning the pull-down assay. A positive and negative control for active or inactive Rho was also performed in parallel. Lysates of cells were preincubated with nonhydrolyzable GTPγS (positive Rho activation) or GDP (negative Rho activation) prior to undergoing precipitation by Rhotekin. Similar levels of active Rho were detected in cells grown on LPA and in cells incubated with GTPγS. Thus, the result indicated that LPA might stimulate an increase in activated Rho and Y-27632 might inhibit the activation of Rho.
Morphological changes induced by Rho-kinase inhibitor and LPA
The effect of Y-27632 on morphologic changes induced by LPA in serum-starved SMMC-7721 cells was observed under a phase-contrast microscope. In serum starvation, cells formed relatively cohesive colonies, and after addition of LPA, they formed dissociated and scattered lamellipodial extensions at the cells’ edges. Addition of Y-27632 at a concentration of 25 μmol/L or more inhibited these LPA-induced morphologic changes, and SMMC-7721 cells remained cohesive with each other.
Inhibition of LPA-induced motility and invasion by Y-27632
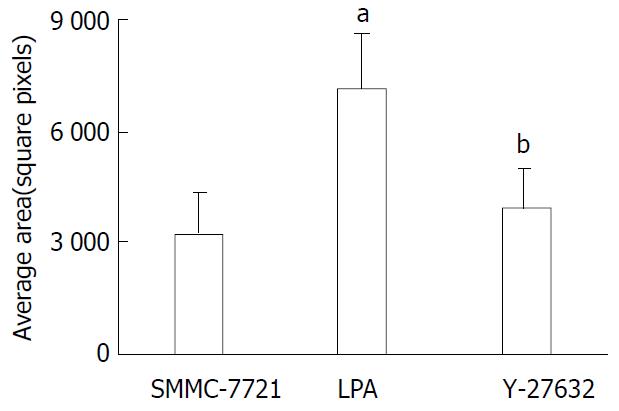
To evaluate the effect of Rho-mediated cellular motility, we assessed the treated cell line in colloidal-gold random motility assay. Cells were seeded onto glass coverslips overlaid with a gold colloid and stimulated with LPA to induce motility. Discernable and quantifiable tracks were left as the cells moved and phagocytized the gold colloid. As determined by the trypan blue dye exclusion assay, the reduction in cell motility was not caused by a decrease in the number of viable cells. At 24 h after stimulation, the cells treated with LPA were 2.2 fold more motile than their untreated counterparts (P < 0.01) and Y-27632 significantly suppressed the LPA-induced motility (Figure 2).
Figure 2 Tumor cell random motility determined by gold-col-loid assay.
Cells were seeded onto the coverslips and allowed to adhere for 1 h and then incubated in FBS containing LPA with or without Y-27632 for 24 h. The areas of clearing in the gold colloid corresponding to phagokinetic cell tracks were counted and represented as -x±s from triplicate experiments. aP < 0.01 LPA treated cells vs SMMC-7721 control cells, bP < 0.01 Y-27632 treated cells vs LPA treated cells.
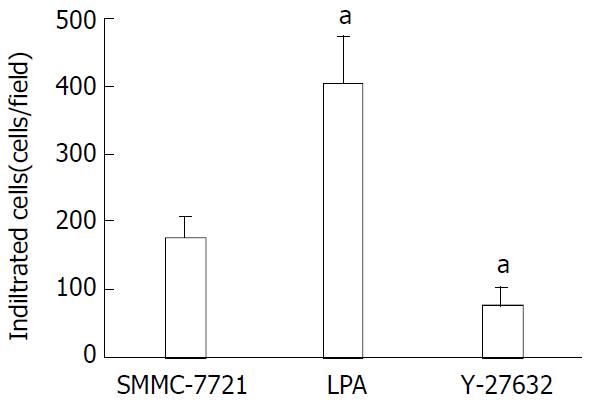
As shown in Figure 3, when the cells were tested for their ability to invade through a matrigel-coated filter in response to a chemoattractant, the LPA-treated cells were 2.3 fold more invasive than the untreated cells. Addition of Y-27632 at a concentration of 25 μmol/L significantly inhibited the LPA-induced invasion (P < 0.01). Taken together, these data suggested that treatment of SMMC-7721 cells with Y-27632 could lead to the inhibition of LPA-mediated motility and invasion.
Figure 3 Inhibition of LPA-induced tumor cell invasion by Y-27632.
Serum-starved cells were seeded onto porous filters. After incubation of the filters with Y-27632 in the presence of LPA 25 μM for 4.5 h to permit penetration of the cells, nonmigrating cells were removed from the upper chamber and migrating cells adherent to the underside of the filters were counted in a minimum of 10-high power fields. Data were expressed as relative migration (number of cells/field) and represented as -x±s from triplicate experiments. aP < 0.01 relative to SMMC-7721 control cells.
DISCUSSION
Generally, metastasis has been found to be a multistep process, which includes detachment of cancer cells from primary tumor, migration, adhesion and invasion of cancer cells into the blood or lymphatic vessels, extravasation out of the vessel, and finally, interactions with the target tissue and the formation of metastastic foci in distant organs[3,21]. In spite of recent advances in diagnostic and therapeutic methods, the prognosis of HCC patients with intrahepatic metastasis was poor[1]. Accordingly, analysis of the mechanism of the migration of cancer cells might lead to new strategies for preventing the progression of hepatocellular carcinoma. The molecular and cellular mechanisms of intrahepatic metastasis have not been fully understood, although cell motility mediated by Rho signaling pathway was recently shown to play a critical role in metastasis of various tumor cells.
Rho is a small GTPase that was found to play an essential role in a variety of cellular processes[6,22]. The active GTP-bound form was involved in cytoskeletal responses to extracellular signaling pathways, resulting in the formation of stress fibres and focal adhesions[23]. Other Rho effects included important roles in signaling pathways that control gene transcription, cell cycle regulation, apoptosis, and tumor progression[24]. Rho proteins could oscillate between an active GTP-bound state and inactive GDP-bound state. The ratio between these two forms is regulated by two classes of proteins: GTPase-activating proteins (GAPs), which promote hydrolysis of GTP bound to the active form and accumulation of the inactive GDP-bound form, and guanine nucleotide exchange factors (GEFs), which promote the exchange of GDP for GTP and therefore induce accumulation of the activated GTP-bound form. In resting cells, inactive GDP-bound Rho proteins were found in the cytosol in complex with another class of regulatory proteins, nucleotide dissociation inhibitors (RhoGDIs). Upon activation, dissociation of RhoGDIs from the complex was required to enable interaction with GEFs and exchange of GDP for GTP[25,26]. To exert their function, activated Rho proteins need to associate with the cell membrane and this in turn depends on their posttranslational modification.
It has been demonstrated that the role of Rho in inducing the invasion could be substituted for by one of its downstream effectors, the Rho-associated kinase (ROCK)[27]. Invasion by tumor cells is a process composed of proteolytic degradation of extracellular matrix barriers and the migration of the cells through the modified region. Contraction of the actomyosin system is important for cell migration, and ROCK regulates the myosin light chain (MLC) phosphorylation by direct phosphorylation of MLC and by inactivation of myosine phosphatase, which was found to result in an increase in actomyosin-based contractility[28,29]. Lysophosphatidic acid is a simple phospholipid that nonetheless has the properties of an extracellular growth factor, mediating diverse cellular responses through the activation of multiple signal transduction pathways. LPA could trigger actin-cytoskeletal events through Rho GTPases, thereby influencing transcellular migration[30]. LPA could induce MLC phosphorylation through Rho activation, leading to the stimulation of cell contractility and motility of fibroblasts and cancer cells[31].
Overexpression of Rho family members increases migration and invasion in various cell lines and culture models, this suggests that such an overexpression in carcinomas might profoundly affect metastatic potential in vivo[32]. The result of Western blot showed that the level of activation of Rho was augmented by LPA. In our study, we extended this observation by providing the evidence that a specific inhibitor of Rho kinase, Y-27632, could inhibit LPA induced Rho activation by GTP-bound Rho pull-down assay. The Rho pathway through a G protein-coupled receptor appeared to be an important process of LPA-mediated signaling.
Y-27632 had an excellent selectivity to inhibit Rho-kinase as compared to other kinases, such as protein kinase C and myosin light-chain kinase in a cell-free system[33]. Thus, Y-27632 is a powerful tool to investigate the role of the Rho/ Rho-kinase system, especially in vivo, although more potent Rho-kinase inhibitors have been synthesized[34]. Y-27632 was reported to inhibit transcellular invasion and reduce the extent of local peritoneal metastases by MM1 rat hepatoma and also in vivo dissemination of prostate cancer cells[15]. In the present study, Y-27632 blocked LPA -mediated SMMC-7721 cell transmonolayer invasion, suggesting the essential role of Rho– Rho kinase pathway in LPA -mediated cell migration. We also observed that Y-27632 induced morphological changes in SMMC-7721 cells, characterized by remaining relatively cohesive colonies, suggesting that Rho-kinase was necessary as a downstream effector of Rho for cell morphologies. Previous reports also demonstrated that Y-27632 inhibited LPA-induced morphologic changes in various cell lines in vitro.
As cell migration, invasion, cellular proliferation, and survival are advantageous to tumor cells for the formation of metastases, the finding that invasiveness of hepatocellular carcinoma is facilitated by the Rho/Rho-kinase pathway is likely to be relevant to tumor progression and the Rho/Rho-kinase may be useful as a prognostic indicator and also in the development of novel therapeutic strategies. Y-27632 may be a new potentially effective agent for the prevention of intrahepatic extension of human HCC.