Published online Sep 1, 2004. doi: 10.3748/wjg.v10.i17.2560
Revised: October 4, 2003
Accepted: October 7, 2003
Published online: September 1, 2004
AIM: To construct a recombinant strain which expresses BabA of Helicobacter pylori (H pylori) and to study the immunogenicity of BabA.
METHODS: BabA2 DNA was amplified by PCR and inserted into the prokaryotie expression vector pET-22b (+) and expressed in the BL21 (DE3) E.coli strain. Furthermore, BabA immunogenicity was studied by animal test.
RESULTS: DNA sequence analysis showed the sequence of BabA2 DNA was the same as the one published by GenBank. The BabA recombinant protein accounted for 34.8% of the total bacterial protein. The serum from H pylori infected patients and Balb/c miced immunized with BabA itself could recognize rBabA.
CONCLUSION: BabA recombinant protein may be an potential vaccine for control and treatment of H pylori infection.
-
Citation: Bai Y, Zhang YL, Chen Y, Jin JF, Zhang ZS, Zhou DY. Cloning and expression and immunogenicity of
Helicobacter pylori BabA2 gene. World J Gastroenterol 2004; 10(17): 2560-2562 - URL: https://www.wjgnet.com/1007-9327/full/v10/i17/2560.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i17.2560
Helicobacter pylori (H pylori), a human-specific gastric pathogen, was first isolated in 1982 and has emerged as the causative agent of chronic active gastritis and peptic ulcer disease[1-11]. Most infected individuals show no clinical symptoms, implicating additional factors, such as genetic predisposition and genotype of the infecting strain, are involved in disease pathogenesis. Chronic infection is associated with the development of gastric adenocarcinoma, one of the most common types of cancer in humans, and H pylori was recently defined as a class 1 carcinogen. In addition, seroprevalence studies indicate that H pylori infection is also associated with cardiovascular, respiratory, extra-gastroduodenal digestive, autoimmune disease. At present the main treatment to eradicate H pylori is combined antibiotics, but the cost of combination therapy and the emergency of antibiotic resistance provide further incentives for vaccine development.
In the development of H pylori vaccine, candidate vaccine antigens adopted currently such as urease, vacuolating cytotoxin, catalase, etc. basically focus on blocking toxicity factors of H pylori while considerably few candidate vaccine antigens focus on adhesins which are closely associated with H pylori colonizing human gastric mucosa by adhering to mucous epithelial cells and mucus layer lining the gastric epithelium. BabA is the only adhesin gene whose receptor has been confirmed. Studies indicate that BabA gene contains two alleles: BabA1 and BabA2. Gene BabA1 is different from BabA2 in the presence of a 10 bp repeat motif and in lack of Leb antigen-binding activity. No concerned study has been reported to date about BabA2 in China. So in this study, recombinant plasmid of H pylori BabA2 gene was constructed and expressed for development of H pylori vaccine.
Bacterial strain BL21 (DE3) and plasmid pET-22 b (+) were provided by Institute of Biotechnology, Academy of Military Medical Sciences. H pylori SS1 was preserved in this research institute. Restriction enzyme Not I, Nco I and T4 DNA ligase, Vent DNA polymerase, isopropyl-β-D-thiogalactopyranoside (IPTG) were purchased from New England Biolabs Company. DNA molecular weight standard λ DNA/EcoRI + HindIII, goat anti-mouse and goat anti-human IgG-HRP were purchased from Huamei Bioengineering Company and His-Tag precolumn from Invitrogen Company. Specific-pathogen-free, female BALB/c mice were housed according to Health Research Council of China guidelines and allowed free access to food and water. Eight H pylori positive sera (which were detected positive by the urease test, pathological dying and germiculture) and three H pylori negative sera (which were detected negative by the above-mentioned three examinations) were from patients treated in the Endoscope Center of this institute. Other reagents were analytically pure reagents produced in China.
Unless otherwise stated, plasmid and chromosomal DNA extractions, resctriction enzyme digests, DNA ligations, transformations into E.coli and other common DNA manipulations were performed by standard procedures. Genome of H pylori was prepared from the cells collected from the colonies on the agar plate. The gene of H pylori BabA2 was amplified from the genome of H pylori by PCR using the primers BabA21 (5’-TG GCC ATG GAT AAA AAA CAC ATC CTT TCA-3’) as upstream primer and BabA22 (5’-AG TGC GGC CGC ATA AGC GAA CAC ATA G-3’) as downstream primer as described in the literature[3]. BabA21 and BabA22 contained Nco I and Not I sites, respectively. PCR was performed with the hot start method. PCR condition was that after initial denaturing at 95 °C for 30 s, each cycle of amplification consisted of denaturing at 95 °C for 30 s, annealing at 55 °C for 30 s and polymerization at 72 °C for 60 s and further polymerization for 10 min after 35 PCR cycles. The PCR product was harvested from agarose gel, digested with Nco I and Not I, and inserted into the Nco I and Not I restriction fragment of the expression vector pET-22b (+) using T4 DNA ligase. The resulting plasmid pET- BabA was transformed into competent E.coli BL21 (DE3) cells using ampicillin resistance for selection. The alkaline lysis method was chosen for large-scale preparations of recombinant plasmids which were identified by restriction enzymes. DNA sequence was performed with a DNA automatic sequencer.
The recombinant strains were incubated overnight at 37 °C and shaked in 5 mL LB with 100 μg/mL ampicillin. A 50 mL LB was inoculated and the cells grew until the optical density at 600 nm reached 0.4-0.6. Isopropyl-β-D-thiogalactopyranoside (IPTG) was added to a final concentration of 0.1, 0.2, 0.4, 0.6, 0.8, 1.0 mmol/L, respectively. E.coli cells from a 50 mL growth 3 h or 5 h after induction were harvested by centrifugation at 12000 g for 10 min and the pellet was resuspended in 1 mL 30 mmol/L Tris buffer (pH8.0) containing 1 mmol/L EDTA (pH8.0), 200 g/L sucrose. The suspension was put on ice for 10 min, and then centrifuged for 10 min at 12000 g, and the resulting suspernatant contained proteins from the periplasm. The resulting pellet was resuspended in 5 mL 50 mmol/L Tris buffer (pH8.0) containing 2 mmol/L EDTA, 0.1 mg/mL lysozyme and 10 g/L Triton X-100. The suspension was incubated at 30 °C for 20 min and then sonicated on ice until it became clarified. The lysate was centrifuged at 12000 g for 15 min at 4 °C, then the resulting supernatant containing proteins from the cytoplasm was purified with Ni-NTA column. Whole-cell lysates, sonicated supernatant,osmotic shock liquid of recombinant strains expressing H pylori BabA2 genes and the purified rBabA were analyzed by electrophoresis analysis in a 100 g/L polyacrylamide gel.
Sixty-eight-week-old mice were immunized 4 times by hypodermic injection in the back of mice at weekly intervals. Each dose consisted of 20 μg of H pylori rBabA protein and 200 μg of adjuvant aluminum hydroxide gel. Age-matched control mice were not immunized. Four weeks after the last immunization, blood samples were taken from the retro-orbital sinus to measure the anti BabA systemic immune responses and stored at -20 °C until assay.
Indirect ELISA as described previously evaluated serum samples from mice and patients for BabA-specific IgG. Purified H pylori rBabA was used as the coating antigen in ELISA immunoassays.
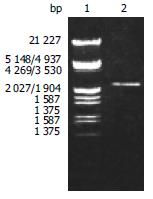
According to the literature, the gene encoding the BabA protein, was amplified by PCR with chromosomal DNA of H pylori Sydeny strain (ss1) as the templates. The cloning products were electrophoresed and visualized on 8 g/L agarose gel (Figure 1). It revealed that BabA2 DNA fragment amplified by PCR contained a gene of approximately 2226 nucleotides, which was compatible with the previous reports.
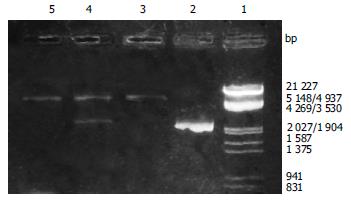
After the PCR products and pET-22 b (+) plasmid were cut by Not I and Nco I, directional cloning was performed, resulting in a recombinant plasmid named pET-22 b (+)/BabA. The recombinant plasmids pET-22 b (+)/BabA were all digested by Not I or Nco I, and by Not I and Nco I simultaneously, then digestive products were visualized on 8 g/L agarose gel eletrophoresis (Figure 2). It demonstrated that recombinant plasmid contained the objective gene.
The nucleotide sequence of cloned genes inserted in pET22 b (+) was analyzed by automatic sequencing across the cloning junction, using the universal primer T7. The results showed that cloned genes contained 2226 nucleotides with a promoter and a start codon coding a putative protein of 741 amino acid residues with a calculated molecular mass of BabA. As compared with previously reports, the homogeneity was 100% between them.
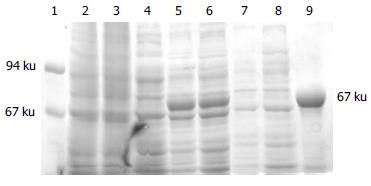
Whole-cell lysate, sonicated supernatant, osmotic shock liquid of recombinant strains expressing H pylori BabA2 genes and the purified rBabA were analyzed by electrophoresis analysis in a 100 g/L polyacrylamide gel for detection of fusion proteins (Figure 3). The result showed that the clearly identifiable band was 78000 u highly expressed fusion proteins, which was similar to that predicted. Gel automatic scan analysis showed that it was 0.6 at D value and the final concentration of IPTG was 0.1 mmol/L and the expression of BabA rose remarkably after 5 h of induction, which accounted for 34.8% of total bacterial proteins. Among them, soluble substance accounted for 15.0% of supernatant. BabA was further analyzed with Ni-NTA column and finally BabA whose purity was 90%.
The postive results by the test of ELISA had colours, but the negtive results had no colours, or weak colours. Serum from the eight mice serums immuned with rBabA showed positive results. In contrast, serum from the eight mice in the control group showed negtive results. At the same time, serums from patients also showed the same results. H pylori positive serum from the eight patients showed positive results and H pylori negative serum from the three patients showed postive results. It showed that anti-BabA antibody existed in the serum of infected patients and rBabA could enable the organism to generate specific humoral immunity.
At first Wadstr et al[9] suspected that the resting or slow growing cells of H pylori could interact with Lewis blood group substances in gastric mucin layer and on epithelium to facilitate initial colonization. Afterwards Iiver et al[7] used receptor activity-directed affinity tagging to purify a kind of adhesin which mediated adherence of H pylori to human gastric epithelial cells by interacting with fucosylated Lewis b (Leb) histo-blood group antigen and named the adhesion BabA. Immunogold electron microscopy found BabA adhesin was located on the bacterial cell outer membrane by probing the Leb antigen. Receptor displacement analyses showed that receptor-adhesin complex was formed under conditions of equilibrium. Most of the cells (> 90%) of the bacterial exhibited BAB activity, as determined by confocal microscopy and fluorescent Leb antigen. The Ka value for formation of the Leb antigen-BabA complex was -1 × 1010 mol/L. The number of Leb glycoconjugate molecules bound to BabA was calculated as -500 per bacterial cell. In addition, the prevalence of blood group antigen-binding activity was also assessed among 95 recent clinical isolates of H pylori, and 66% (63 isolates) were bound to the Leb antigen. Bosch et al[11] investigated the effects of acute stress on the salivary levels of the carbohydrate structure sulfo-Lewis (sulfo-Le). The results showed the stressor induced a strong increase in salivary sulfo-Le concentration (U/mL), sulfo-Le output (U/min), sulfo-Le/total protein ratio (U/mg protein), and saliva-mediated adherence (ex vivo) of H pylori. As expected, sulfo-Le concentration correlated with the adherence of H pylori (r = 0.72, P < 0.05). In a word, the adhesin BabA and its receptor Leb played an important role in the adherence mechanism of H pylori, especially when people were in the state of acute stress.
Isolates of H pylori differed in virulence and from individuals with peptic ulcers most often were type I strains that expressed vacuolating cytotoxin A (VacA) and cytotoxin-associated gene A (CagA) protein. By definition, type II strains could express neither marker. The bacterial Leb-binding phenotype was associated with the presence of the cag pathogenicity island among clinical isolates of H pylori. A vaccine strategy based on the BabA adhesin might serve as a means to target the virulent type I strains of H pylori. So we selected H pylori ss1 chromosome DNA as a template, which has strong adhesin and is capable of coloning firmly in the stomach of a mouse, designed specific primers and successfully screened the genes of BabA2. To facilitate the expression and purification at the next stage, we cloned it into a fusion expression vector with 6 hercynine tails. The restriction enzyme digestion and the sequencing results showed that one open reading frame had the size of 2226 bp. SDS-PAGE and scan analysis showed that the molecular mass of BabA was 78 ku and recombinant protein accounted for 34.8% of the total bacterial protein. Considering that the recombinant protein with bio-activity tended to preserve more integral and positive antigenicity, we selected supernatant of ultrasound disrupted live E.coli and performed repeated purification of rBabA with Ni-NTA resin, thus obtaining rBabA with activity whose purity was 90%. This has laid a foundation for further research on immune protection and molecular adhesin mechanism. In this study, serum from the mice immuned with purified recombinant protein BabA could be used to detect specific antibody against BabA, but serum from the mice in the control group could not. Serum from patients also showed the same results. These results suggest that BabA of H pylori maybe a good candidate as a vaccine component.
Edited by Hu DK and Wang XL Proofread by Xu FM
| 1. | Bai Y, Zhang YL, Wang JD, Lin HJ, Zhang ZS, Zhou DY. Conservative region of the genes encoding four adhesins of Helicobacter pylori: cloning, sequence analysis and biological information analysis. Di Yi Jun Yi Da Xue Xue Bao. 2002;22:869-871. [PubMed] |
| 2. | Vandenplas Y. Helicobacter pylori infection. World J Gastroenterol. 2000;6:20-31. [PubMed] |
| 3. | Bai Y, Chang SH, Wang JD, Chen Y, Zhang ZS, Zhang YL. Construction of the E.coli clone expressing adhesin BabA of Helicobacter pylori and evaluation of the adherence activity of BabA. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:293-295, 309. [PubMed] |
| 4. | Morgner A, Miehlke S, Stolte M, Neubauer A, Alpen B, Thiede C, Klann H, Hierlmeier FX, Ell C, Ehninger G. Development of early gastric cancer 4 and 5 years after complete remission of Helicobacter pylori associated gastric low grade marginal zone B cell lymphoma of MALT type. World J Gastroenterol. 2001;7:248-253. [PubMed] |
| 5. | Bai Y, Zhang YL, Wang JD, Zhang ZS, Zhou DY. [Construction of the non-resistant attenuated Salmonella typhimurium strain expressing Helicobacter pylori catalase]. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:101-105. [PubMed] |
| 6. | Solnick JV, Canfield DR, Hansen LM, Torabian SZ. Immunization with recombinant Helicobacter pylori urease in specific-pathogen-free rhesus monkeys (Macaca mulatta). Infect Immun. 2000;68:2560-2565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, Incecik ET, Berg DE, Covacci A, Engstrand L, Borén T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 842] [Article Influence: 31.2] [Reference Citation Analysis (1)] |
| 8. | Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd editor. New York: Cold Spring Harbor Laboratory Press 1989; 35-400. |
| 9. | Wadström T, Hirmo S, Borén T. Biochemical aspects of Helicobacter pylori colonization of the human gastric mucosa. Aliment Pharmacol Ther. 1996;10 Suppl 1:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Bosch JA, de Geus EJ, Ligtenberg TJ, Nazmi K, Veerman EC, Hoogstraten J, Amerongen AV. Salivary MUC5B-mediated adherence (ex vivo) of Helicobacter pylori during acute stress. Psychosom Med. 2000;62:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791-5795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 933] [Article Influence: 29.2] [Reference Citation Analysis (0)] |