Published online Aug 15, 2004. doi: 10.3748/wjg.v10.i16.2394
Revised: November 21, 2003
Accepted: December 16, 2003
Published online: August 15, 2004
AIM: To investigate whether the non-invasive real-time Indocynine green (ICG) clearance is a sensitive index of liver viability in patients before, during, and after liver transplantation.
METHODS: Thirteen patients were studied, two before, three during, and eight following liver transplantation, with two patients suffering acute rejection. The conventional invasive ICG clearance test and ICG pulse spectrophotometry non-invasive real-time ICG clearance test were performed simultaneously. Using linear regression analysis we tested the correlation between these two methods. The transplantation condition of these patients and serum total bilirubin (T. Bil), alanine aminotransferase (ALT), and platelet count were also evaluated.
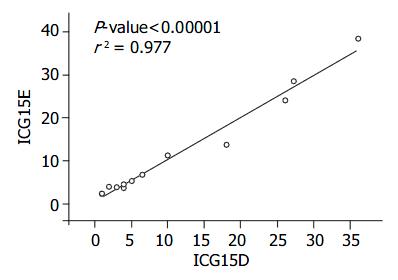
RESULTS: The correlation between these two methods was excellent (r2 = 0.977).
CONCLUSION: ICG pulse spectrophotometry clearance is a quick, non-invasive, and reliable liver function test in transplantation patients.
- Citation: Hsieh CB, Chen CJ, Chen TW, Yu JC, Shen KL, Chang TM, Liu YC. Accuracy of indocyanine green pulse spectrophotometry clearance test for liver function prediction in transplanted patients. World J Gastroenterol 2004; 10(16): 2394-2396
- URL: https://www.wjgnet.com/1007-9327/full/v10/i16/2394.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i16.2394
Monitoring of liver function is important in patients undergoing liver transplantation. Exact liver function and the extent of the remaining liver function at end-stage liver disease can indicate the appropriate timing of liver transplantation[1-3]. During liver transplantation, immediate liver function evaluation can resolve primary non-functioning of the liver and technical complications[4]. Moreover, post-transplantation early liver function evaluation can detect delayed graft malfunction or acute/chronic graft rejection.
Indocyanine green (ICG) is a synthetic dye that has been used for many years to measure hepatic blood flow and as a test of liver function[5]. It was also used by Makuuchi and his colleagues, based on their experience, as a guide for selecting the type of resection to be performed[6,7]. During liver transplantation, ICG clearance rate has been used for evaluation of the recipient liver[3,8], the donor liver[9,10] and the post-transplant liver graft[3]. Conventional ICG clearance is determined by measuring the rate of elimination using 3 mL venous samples at 0, 5, 10, 15, and 20 min after administering ICG. This invasive technique produces patient discomfort through repeated peripheral venous sampling at exact time intervals. Pulse spectrophotometry is an infrared-based non-invasive technique originally developed for monitoring tissue oxygenation. It has been previously used for the measurement of hepatic ICG concentration by Shinohara et al[11]. In a recent case report, infrared spectrophotometry was sufficiently sensitive to measure the ICG clearance in surgical patients[12].
Even though the ICG clearance test has been used worldwide to predict liver function, there are some limitations for end-stage liver disease in patients with hyperbilirubinemia, intrahepatic shunt or capillarization[13].
ICG pulse spectrophotometry (LiMON) has not been tested in transplanted patients previously.
The aim of this study was to determine the accuracy of ICG spectrophotometry, compared with the conventional invasive method, in predicting liver function in waiting and transplanted patients.
Thirteen patients who underwent liver transplantation and follow-up at the Tri-Service General Hospital were evaluated in the current study. Eleven male patients were transplanted for chronic liver disease of various etiologies (1 hepatitis C, 2 hepatitis B, and 8 hepatitis B with hepatoma) and 2 patients with end-stage liver disease awaiting transplantation. Patients 7 and 11 were studied in acute rejection during follow up (d36 and 90) and proved by liver biopsy. Patients 3, 4 and 13 were studied immediately post-liver transplantation (within 24, 27 and 12 h ) (Table 1).
| Diagnosis | Condition (d) | T. Bil | ALT | Cr. | PLT | ICG15D | ICG15E | ICG(K)D | ICG(K)E | |
| 1 | Alcoholism | Waiting | 1.6 | 121 | 0.8 | 16.4 | 27.2 | 28.4 | 0.05 | 0.08 |
| 2 | HBV+HCC | Waiting | 1.4 | 47 | 0.9 | 12.6 | 36 | 38.3 | 0.03 | 0.06 |
| 3 | HBV | During-OLT-(2) | 1.6 | 126 | 0.8 | 7.4 | 3 | 3.9 | 0.24 | 0.22 |
| 4 | HBV | During-OLT-(2) | 1.1 | 102 | 1.0 | 11.8 | 1 | 2.5 | 0.28 | 0.29 |
| 5 | HCV+HCC | Post-OLT (43) | 0.7 | 233 | 2.3 | 15.1 | 4 | 3.7 | 0.18 | 0.22 |
| 6 | HBV | Post-OLT (212) | 0.5 | 8 | 2.9 | 12.1 | 1 | 2.3 | 0.35 | 0.29 |
| 7 | HBV | Post-OLT (36) | 9.1 | 131 | 1.6 | 7.2 | 26 | 24 | 0.10 | 0.10 |
| Ac. rejection | ||||||||||
| 8 | HBV+HCC | Post-OLT (142) | 0.8 | 15 | 1.3 | 11 | 6.5 | 6.8 | 0.21 | 0.25 |
| 9 | HBV+HCC | Post-OLT. (166) | 1.1 | 37 | 0.7 | 24.9 | 2 | 4 | 0.32 | 0.23 |
| 10 | HBV+HCC | Post-OLT (312) | 0.5 | 16 | 1.0 | 18.8 | 4 | 4.5 | 0.24 | 0.21 |
| 11 | HBV+HCC | Post-OLT (90) | 2.0 | 50 | 2.2 | 11.5 | 18 | 13.7 | 0.16 | 0.13 |
| Ac. rejection | ||||||||||
| 12 | HBV+HCC | Post-OLT (367) | 1.3 | 46 | 3.4 | 6.4 | 10 | 11.2 | 0.28 | 0.27 |
| Ac. renal failure | ||||||||||
| 13 | HBV+HCC | During OLT (1) | 3.6 | 213 | 3.4 | 8.5 | 5.1 | 5.4 | 0.31 | 0.33 |
An intravenous (IV) bolus of 0.5 mg/kg ICG was injected rapidly through a central venous catheter or large peripheral venous line, and samples were obtained from another peripheral venous line at 0, 5, 10, 15, 20 min thereafter and kept in an EDTA-treated tube at room temperature until centrifuged at 3000 r/min for 10 min. Absorbance was measured by a Perkin Elmer spectrophotometer at 805 nm. Direct ICG retention rate at 15 min (ICG15D) and the elimination rate constant (ICG(K)D) values were calculated using a commercial computer program (V-500 Spectra Manager, Jasco, Japan).
Pulse-dye densitometry (LiMON, Stahigruburring, Munich, Germany) was used to measure the blood ICG concentration non-invasively in real time. This apparatus makes such measurements possible by continuously monitoring the optical absorption at 805 nm and 890 nm, via an optical probe attached to the patient’s finger. During the first 5-10 min after ICG was injected, blood ICG concentrations were monitored at every pulse interval via pulse spectrophotometry. The elimination rate constant ICG(K)E was calculated automatically by the time course of blood ICG concentration. The estimated ICG15 retention rate (ICG15E ) was obtained via pulse spectrophotometry computer analysis during the first 5-10 min. As the usual measurement time of the Limon is between 5 and 10 min, the (ICG15E) is simply calculated from the ICG plasma disappearance rate[14].
Linear regression analysis was used to test the correlation. Statistical significance was considered at P < 0.05. The statistic software was S-Plus® 2000 for Windows (CANdiensten, Amsterdam, The Netherlands).
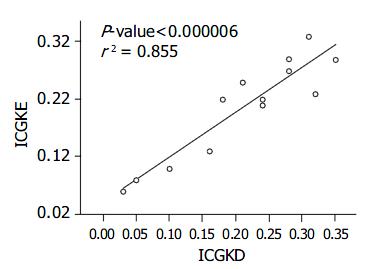
The correlation between ICG15D, ICG15E, ICG(K) D, ICG(K) E values for ICG15 and ICG(K) by these two methods was excellent (r2 = 0.977, 0.855) (Figure 1, Figure 2, Table 2). Two patients had poor liver function, elevated T. Bil, and higher ICG15 retention rates, and were on the waiting list for transplantation. Three patients who had good liver function immediately post-liver transplantation showed elevated T. Bil. and lower ICG15 retention rates. Two patients who were in acute rejection had poor liver function with elevated ALT and higher ICG15 retention rates. Five patients, who were in a stable condition with good liver function post-liver transplantation, had low T. Bil. and better ICG15 retention rates.
| Patients condition (n) | T. Bil | ICG15D | ICG15E | ICG(K)D | ICG(K)E |
| ESLD (2) | 1.50 ± 0.10 | 29.6 ± 4.4 | 34.4 ± 4.9 | 0.04 ± 0.01 | 0.07 ± 0.01 |
| During-OLT (3) | 1.35 ± 0.25 | 2.0 ± 1.0 | 3.2 ± 0.7 | 0.26 ± 0.02 | 0.25 ± 0.04 |
| Post-OLT (6) | 0.86 ± 0.30 | 3.6 ± 2.1 | 3.9 ± 0.8 | 0.26 ± 0.08 | 0.24 ± 0.03 |
| Acute rejection (2) | 5.60 ± 3.60 | 22.0 ± 4.0 | 18.9 ± 5.2 | 0.13 ± 0.03 | 0.12 ± 0.02 |
ICG is a water-soluble tricarbocyanine dye that is extracted by hepatic parenchyma cells and excreted almost entirely into bile. Hepatic clearance of ICG occurs by two major processes: by uptake across the sinusoidal plasma membrane with a high extraction ratio; and by removal from the hepatocytes via cytoplasmic transport and exclusive biliary excretion with neither intrahepatic conjugation nor enterohepatic circulation[15]. The concentration in the plasma can be measured by spectrophotometry and its rate of elimination has been widely used as a measure of liver blood flow and liver function[16]. Most of the studies assessing liver function before hepatectomy and the postoperative outcome were carried out using the ICG clearance test[17].
Pulse spectrophotometry has been developed in recent years to enable the blood ICG concentration to be measured easily, less invasively, and continuously. The previous method for measuring the ICG concentration by spectrophotometry did not show sufficient accuracy. The new approach, based on pulse spectrophotometry, is able to continuously measure the arterial ICG concentration because of its ability to detect the pulse wave, greatly improving its accuracy.
Pulse spectrophotometry measurements of ICG concentration with each pulse could provide information on ICG liver uptake and excretion. From the concentration, a time curve of the ICG uptake and excretion rates were calculated under different experimental conditions. The experimental study by El-Desoky et al[5] showed the sensitivity of measurement of ICG uptake and excretion rates in hepatic artery occlusion, portal vein occlusion, ischemia-reperfusion injury, hepatic microcirculation, colchicines treatment, and bile duct ligation.
Assessment of liver function remains difficult in transplanted patients because of etiological complexity. The differential diagnosis includes primary non-function of the graft, rejection, virus re-infection, drug intoxication, and thrombosis of hepatic blood vessels. After liver transplantation, liver function tests are often difficult to interpret and non-specific. The diagnosis at present relies on a combination of biochemical, hemodynamic, clinical markers, and occasionally liver biopsy. However, serial observations up to 72 h are required. Measurement of ICG clearance appears to be a simple and safe test to assess early liver graft function. The development of non-invasive pulse spectrophotometry has enabled bedside assessment of the elimination of ICG with immediate results, therefore increasing its clinical usefulness.
A study by Jalan et al showed the ICG measured at 24 h after liver transplantation accurately reflected graft function and predicted graft survival and outcome. In other studies, ICG excretion was a sensitive index of ischemia/reperfusion injury during the early stages post-liver transplantation[17].
The traditional ICG clearance test is invasive and labor-dependent. The whole procedure requires more than 30 min at the bedside for loading dosage and venous puncture. Failure may occur as a result of the patient’s underlying disease and medication such as gout, arthritis, and anti-TB drugs. Technical failure may be due to blood hemolysis or laboratory errors. The real-time pulse spectrophotometry ICG clearance test is non-invasive and machine-dependent. Technical failure may be due to detecting probe malpositioning, and patient movement.
In this study, we have chosen two ICG clearance test methods to measure end-stage liver disease, immediately post-liver transplantation or in acute rejection patients. In patients with hyperbilirubinemia, before and after successful liver transplantation, the ICG15 clearance test may be very useful in evaluating the new liver’s function. ICG pulse spectrophotometry appears to be a sensitive and specific test to predict graft function in ischemia/reperfusion and acute rejection patients. The correlations between ICG(K) and ICG15 using these two methods were excellent.
In conclusion, in this study, the correlation between conventional and pulse spectrophotometry ICG clearance tests is excellent in transplanted patients. The ICG pulse spectrophotometry clearance test is a quick, non-invasive, easy, inexpensive, and reliable liver function test in transplantation patients.
Edited by Zhu LH Proofread by Xu FM
| 1. | Miyagawa S, Makuuchi M, Kawasaki S, Kakazu T. Criteria for safe hepatic resection. Am J Surg. 1995;169:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 263] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Oellerich M, Burdelski M, Lautz HU, Rodeck B, Duewel J, Schulz M, Schmidt FW, Brodehl J, Pichlmayr R. Assessment of pretransplant prognosis in patients with cirrhosis. Transplantation. 1991;51:801-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Tsubono T, Todo S, Jabbour N, Mizoe A, Warty V, Demetris AJ, Starzl TE. Indocyanine green elimination test in orthotopic liver recipients. Hepatology. 1996;24:1165-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Plevris JN, Jalan R, Bzeizi KI, Dollinger MM, Lee A, Garden OJ, Hayes PC. Indocyanine green clearance reflects reperfusion injury following liver transplantation and is an early predictor of graft function. J Hepatol. 1999;30:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | El-Desoky A, Seifalian AM, Cope M, Delpy DT, Davidson BR. Experimental study of liver dysfunction evaluated by direct indocyanine green clearance using near infrared spectroscopy. Br J Surg. 1999;86:1005-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Lau H, Man K, Fan ST, Yu WC, Lo CM, Wong J. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997;84:1255-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 196] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Parks RW, Garden OJ. Liver resection for cancer. World J Gastroenterol. 2001;7:766-771. [PubMed] |
| 8. | Niemann CU, Yost CS, Mandell S, Henthorn TK. Evaluation of the splanchnic circulation with indocyanine green pharmacokinetics in liver transplant patients. Liver Transpl. 2002;8:476-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Seifalian AM, Mallet SV, Rolles K, Davidson BR. Hepatic microcirculation during human orthotopic liver transplantation. Br J Surg. 1997;84:1391-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Koneru B, Leevy CB, Klein KM, Zweil P. Clearance of indocyanine green in the evaluation of liver donors. Transplantation. 1994;58:729-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Shinohara H, Tanaka A, Kitai T, Yanabu N, Inomoto T, Satoh S, Hatano E, Yamaoka Y, Hirao K. Direct measurement of hepatic indocyanine green clearance with near-infrared spectroscopy: separate evaluation of uptake and removal. Hepatology. 1996;23:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Mandell MS, Wachs M, Niemann CU, Henthorn TK. Elimination of indocyanine green in the perioperative evaluation of donor liver function. Anesth Analg. 2002;95:1182-114, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Ott P, Clemmesen O, Keiding S. Interpretation of simultaneous measurements of hepatic extraction fractions of indocyanine green and sorbitol: evidence of hepatic shunts and capillarization? Dig Dis Sci. 2000;45:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Sakka SG, Meier-Hellmann A. Non-invasive liver function moni-toring by indocyanine green plasma disappearance rate in critically ill patients. International J Intensive Care. 2002;66-72. |
| 15. | WHEELER HO, CRANSTON WI, MELTZER JI. Hepatic uptake and biliary excretion of indocyanine green in the dog. Proc Soc Exp Biol Med. 1958;99:11-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | CAESAR J, SHALDON S, CHIANDUSSI L, GUEVARA L, SHERLOCK S. The use of indocyanine green in the measurement of hepatic blood flow and as a test of hepatic function. Clin Sci. 1961;21:43-57. [PubMed] |
| 17. | Kawasaki S, Makuuchi M, Miyagawa S, Kakazu T, Hayashi K, Kasai H, Miwa S, Hui AM, Nishimaki K. Results of hepatic resection for hepatocellular carcinoma. World J Surg. 1995;19:31-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 2.4] [Reference Citation Analysis (0)] |