Published online Jul 15, 2004. doi: 10.3748/wjg.v10.i14.2091
Revised: November 23, 2003
Accepted: December 8, 2003
Published online: July 15, 2004
AIM: To investigate the cytotoxic activity of extracts of trichosanthes root tubers (EOT) on HepA-H cells and HeLa cells compared with trichosanthin (TCS), and to explore the possible mechanism of growth inhibitory effect of EOT on HeLa cells.
METHODS: Tumor cells were cultured in vitro, and then microculture tetrzoalium assay (MTT) was used to investigate drugs’ cytotoxic activity. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to observe ultrastructural changes of cells, and electrophoresis was performed to detect changes of biochemical characteristics of intercellular DNA.
RESULTS: TCS and EOT had no obvious effects on HepA-H cells (P > 0.05), but had remarkable effects on HeLa cells in a time and dose dependent manner (r > 0.864, P < 0.05 or P < 0.01). The inhibitory rate of EOT was much higher than that of TCS (P < 0.01). Median inhibitory rates (IC50) of TCS and EOT on HeLa cells were 610.9 mg/L and 115.6 mg/L for 36 h, and 130.7 mg/L and 33.4 mg/L for 48 h respectively. Marked morphologic changes were observed including microvillus disappearance or reduction, cell membrane bledding, cell shrinkage, condensation of chromosomes and apoptotic bodies with complete membranes. Meanwhile, apoptosis of HeLa cells was confirmed by DNA ladder formation on gel electrophoresis.
CONCLUSION: TCS and EOT have no obvious effects on HepA-H cells, but have significant inhibitory effects on HeLa cells, indicating that EOT is superior to TCS in anti-tumor activity.
- Citation: Dou CM, Li JC. Effect of extracts of trichosanthes root tubers on HepA-H cells and HeLa cells. World J Gastroenterol 2004; 10(14): 2091-2094
- URL: https://www.wjgnet.com/1007-9327/full/v10/i14/2091.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i14.2091
Trichosanthes Kirilowii Maximowii is a kind of liana of the Cucurbitaceae family, whose root tuber was a Chinese herbal medicine Tianhuafen (THF) that has been used to reset menstruation and expel retained placenta[1]. In the 1980 s, Trichosanthin (TCS) was isolated from the root tuber and proved to be the active component, a type I ribosome-inactivating protein (RIP) with 247 amino acids which inactivates eukaryotic ribosomes via its N-glycosidase activity. TCS has been used to induce mid-term abortion and to treat ectopic pregnancies, hydatidiform and trophoblastic moles in China[2]. In recent years, TCS has also been found to posses various pharmacological properties including immunomudulatory, anti-tumor and anti-HIV activities[3-6]. Clinical trials have also been performed. TCS has aroused extensive attention.
In the present study, we were interested in its anti-tumor activity in vitro. TCS has already been regarded as an effective anti-tumor agent highly specific to choriocarcinoma cells from trophoblasts[7]. However, researches and clinical application about TCS on tumor are mainly on gastrointestinal tumor and seldom on others[8]. TCS is an active anti-tumor component of Trichosanthes, yet there is no report of extracts of trichosanthes root tubers (EOT) on tumor cells. So in this study, we chose two tumor cell lines, HepA-H cells and HeLa cells, to investigate the growth inhibitory effect of EOT and TCS on the two cell lines and further compared their anti-tumor activity.
In recent years, screening of anti-tumor drugs from natural resource, especially from traditional Chinese medicines, has drawn worldwide research interest, and many exciting goals have been achieved[9]. As a research focus of life science and medicine, apoptosis has revealed its promising future[10-12]. Nowadays apoptosis has been considered as the most ideal path to conquer tumors. Therefore, in our study, we initially investigated the induction of apoptosis of HeLa cells by EOT. The present study may provide experimental bases for further researches and seeking novel anti-tumor agents.
TCS (1.2 mg/mL) was purchased from Jinshan Pharmacy Ltd, Shanghai. EOT was extracted from fresh root tubers of Trichosanthes kirlowii Maxim collected in Hangzhou[13], Zhejiang Province in November 2002. SDS-PAGE (100 g/L) was performed to detect the components of EOT samples in comparison with TCS and protein marker. Gel was stained by Coomassie brilliant blue. After decolored, the gel was visualized and photographed under gel imaging assay apparatus. Then straps in the gel were scanned to evaluate the content of relative proteins. Drugs were diluted with culture medium and sterilized before use.
RPMI-1640 and fetal calf serum (FCS) were from GIBCO, USA. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), DMSO and all electrophoresis reagents were from Sigma Co., USA. Animal cell PCD ladder isolation kit was purchased from Dingguo Biological Products Ltd, Beijing. CO2 incubator was the product of FORMA Company. TECNAI 10 TEM was from PHILIPS and STEREOSCAN 260 was from CAMBRIDGE Company. Electrophoresis apparatus was made by E-C Apparatus Corporation. Mini gel electrophoresis system was the product of Bio-Rad Company. Image analysis system was purchased from Shanghai Tianneng Science and Technology Ltd.
HepA-H and HeLa cells were preserved in our laboratory. The cell line of high lymphatic metastasis ascitic liver cancer was established and cultivated by professor Li and Yang[14]. Cells were grown in RPMI-1640 culture medium containing 100 mL/L FCS, 100 U/mL penicillin and 100 U/mL streptomycin at 37 °C in a humidified atmosphere of 50 mL/L CO2.
Growth inhibitory effects of HepA-H and HeLa cells with various treatments were determined by MTT assay. Cells at exponential phase were seeded into 96-well plates, 100 μL (1 × 105/mL) per well. Then different concentrations of EOT and TCS in 100 μL culture medium were added and the final concentration in each well was 0.1 mg/L, 1 mg/L, 10 mg/L, 100 mg/L, and 500 mg/L respectively. Each treatment was tested in tetrad wells and the control group was given only culture medium containing no drug. All the above plates were placed in a 50 mL/L CO2 humidified-atmosphere incubator at 37 °C for 24, 36 and 48 h. At the end of exposure, 20 μL MTT (5 g/L ) was added to each well and the plates were incubated at 37 °C for 4 h. Then all culture medium supernatant was removed from wells and replaced with 100 μL DMSO. The plates were shaken for 15 min so that all the production of formazan reduced by MTT could be dissolved out from cells. Following thorough formation dissolved, the absorbance of each well was measured by standard enzyme-linked immunosorbant assay at 492 nm. The inhibition of tumor cell growth was calculated by the equation:
Growth inhibitory rate = (1 - A492 treated/A492 control) × 100%.
Based on the growth inhibitory rates of each group of drugs on cells, SPSS software was used to calculate 50% inhibitory concentrations (IC50).
HeLa cells at exponential phase were used and cultivated with various concentrations of EOT for 24 h. Then cells were harvested and fixed with 25 g/L glutaraldehyde in 0.1 mol/L phosphate buffer (pH7.4) for 2 h at 4 °C. For TEM examination, the specimens were postfixed in 10 g/L osmium tetroxide in 0.1 mol/L phosphate buffer (pH7.4) for 1 h at 4 °C. After dehydration in a graded series of ethanol and infiltration in propylene oxide, they were embedded in Epson 812. Fine sections were cut with an LKB 2088 ultramicrotome and stained with uranyl acetate and lead citrate. The sections were examined with a PHILIPS TECNAI 10 TEM at 60 kV. For SEM examination, the specimens were postfixed for 1 h in 10 g/L OSO4, dehydrated in a graded series of alcohol, CO2 critical-point dried, mounted on aluminum stubs and sputter-coated with gold. Specimens were examined with a STEREOSCAN 260 SEM at 25 kV.
HeLa cells at exponential phase were exposed to different concentrations of EOT for 24, 36 and 48 h respectively. Then cells were harvested, washed twice with PBS, and DNA was extracted according to the directions of the kit. DNA was analyzed by electrophoresis at 75 mA on 8 g/L agarose gel containing 0.5 mg/L ethidium bromide. After about 1.5 h, the gel was visualized and photographed under transmission UV light.
Data were expressed as mean ± SD. Statistics software package SPSS 11.5 was employed to process the data. Differences between groups were described by Student t test. P values less than 0.05 were considered statistically significant.
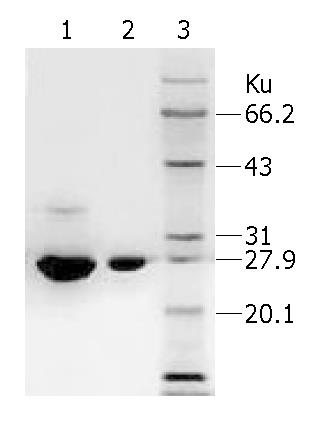
1 kg fresh trichosanthes root tubers was used in the present study and we got 0.83 g EOT. As the result of SDS-PAGE showed, sample EOT had an obvious major strap and several other minor straps. All the straps were scanned, sample EOT had a content of 70.93% of TCS and other unknown proteins. However, sample TCS had only a minor strap and its purity was 99.81%. Protein marker further conformed to protein TCS (Figure 1).
After exposure to EOT and TCS, HepA-H cells had no growth arrest of significant importance. After 24 h, most growth of HepA-H cells was promoted, but the effect did not depend on the dose (P > 0.05). After 36 h, all the cells were led to growth arrest, which also had no statistical relation with the dose. After 48 h, some were promoted, while the others were inhibited, and the relationship between the effect and dose was not found. All the three groups of data showed no statistical significance (P > 0.05), suggesting HepA-H cells were not susceptible to EOT and TCS (Table 1).
EOT and TCS had good effects on HeLa cells in a time and dose dependant manner (r > 0.864). When the concentration of drugs was increased and the time was prolonged, the growth inhibitory rate increased gradually (P < 0.01). EOT was superior to TCS in the effect on HeLa cells when the time and concentration were identical (P < 0.01). The median inhibitory rates (IC50) of TCS and EOT for HeLa cells were 610.9 mg/L and 115.6 mg/L after 36 h, and 130.7 mg/L and 33.4 mg/L after 48 h, respectively. The results suggested that EOT and TCS could inhibit the growth of HeLa cells, while EOT had much stronger cytotoxic activity (Table 2).
| Drug | Concentration (mg/L) | Inhibitory rate (%) | ||
| 24 h | 36 h | 48 h | ||
| EOT | 0.1 | -3.1 ± 2.9 | 19.1 ± 2.6 | 15.7 ± 0.8 |
| 1.0 | -3.6 ± 8.1 | 7.9 ± 11.5 | 5.1 ± 2.6 | |
| 10 | -25.0 ± 1.7 | 6.8 ± 0.1 | 3.8 ± 3.6 | |
| 100 | 9.6 ± 3.3 | 21.0 ± 2.2 | 16.2 ± 6.3 | |
| 500 | 7.6 ± 7.6 | 17.0 ± 3.0 | 13.4 ± 0.7 | |
| TCS | 0.1 | -19.9 ± 6.7 | 6.9 ± 5.2 | -3.7 ± 4.9 |
| 1.0 | -6.1 ± 6.4 | 4.7 ± 0.6 | 7.4 ± 2.7 | |
| 10 | -16.1 ± 19.1 | 0.7 ± 2.4 | -11.4 ± 4.7 | |
| 100 | -6.8 ± 1.0 | 11.9 ± 4.1 | 2.6 ± 2.3 | |
| 500 | -3.4 ± 3.3 | 10.8 ± 5.2 | 5.9 ± 3.8 | |
| Drug | Concentration (mg/L) | Inhibitory rate (%) | ||
| 24 h | 36 h | 48 h | ||
| EOT | 0.1 | 3.4 ± 4.1 | 9.7 ± 7.0 | 9.9 ± 3.1 |
| 1.0 | 4.5 ± 3.7 | 11.1 ± 2.0 | 11.3 ± 1.9 | |
| 10 | 16.3 ± 3.2 | 20.9 ± 6.8 | 29.4 ± 4.1 | |
| 100 | 36.7 ± 4.0 | 44.2 ± 1.7 | 54.6 ± 3.0 | |
| 500 | 57.9 ± 3.4 | 71.8 ± 3.9 | 89.4 ± 1.9 | |
| TCS | 0.1 | 2.2 ± 1.7 | 2.9 ± 2.7 | 4.4 ± 3.1 |
| 1.0 | 2.9 ± 2.0 | 5.5 ± 1.2 | 6.0 ± 2.7 | |
| 10 | 16.4 ± 4.1 | 6.7 ± 2.4 | 13.0 ± 3.3 | |
| 100 | 26.3 ± 2.5 | 29.3 ± 3.9 | 47.9 ± 5.2 | |
| 500 | 39.6 ± 4.8 | 53.8 ± 4.0 | 70.2 ± 2.9 | |
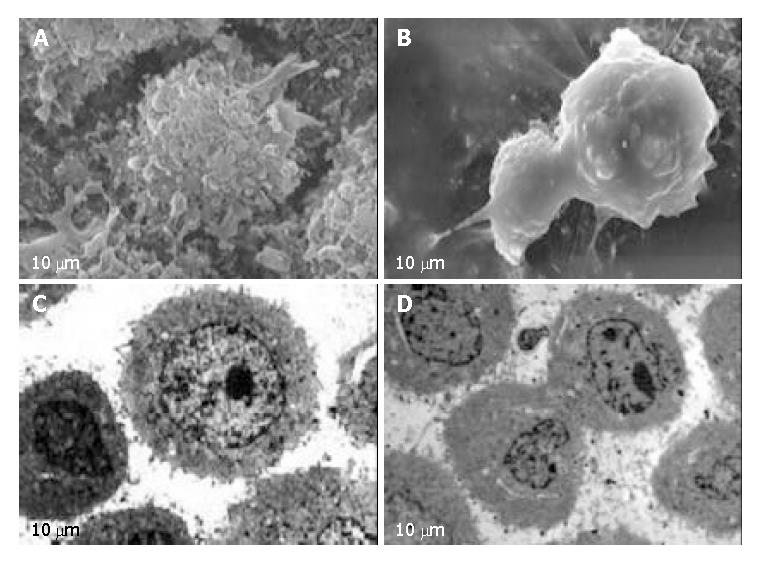
After exposure to EOT for 24 h, HeLa cells were observed under SEM, typical apoptotic characteristics were found, including cell membrane bledding, microvilli disappearance or reduction, and separated apoptotic bodies (Figure 2, A-B). Also treated HeLa cells were observed under TEM, and shrinkage of cells, condensation of chromosomes and apoptotic bodies with complete membrane were found (Figure 2, C-D).
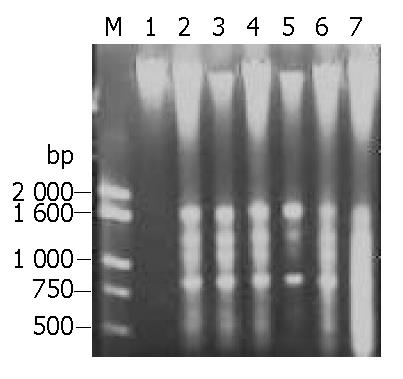
After HeLa cells were exposed to EOT 50 mg/L, 100 mg/L, 500 mg/L respectively for 24 h, DNA agarose gel electrophoresis showed the typical “DNA ladder” pattern of apoptosis. With time delayed, 48 h later, DNA ladder was much more obvious in the cells treated with EOT 50 mg/L, 100 mg/L, but DNA of cells treated with high concentration, 500 mg/L, took on a shape of smear, suggesting that cells underwent secondary necrosis (Figure 3). Results of DNA agarose gel electrophoresis further confirmed that EOT could induce HeLa cell apoptosis.
To our knowledge, researches of the effects of TCS on liver cancer arrived different conclusions, while studies on HeLa cells were scarcely reported[15,16]. In the present work, we investigated and analyzed the effect of EOT and TCS on HepA-H and HeLa cells. We found that TCS and EOT had no significant effect on HepA-H cells but had remarkable effect on HeLa cells in a time and dose dependant manner. The data suggested EOT had a much higher anti-tumor effect on HeLa cells than TCS. After 48 h, the growth inhibitory rate of HeLa cells exposed to EOT and TCS (500 mg/L) was 89.4% and 70.2%, respectively. The median inhibitory rates (IC50) were 610.9 mg/L and 115.6 mg/L after 36 h, and 130.7 mg/L and 33.4 mg/L after 48 h, respectively. SDS-PAGE showed, EOT contains 70.93% TCS and several other components. In contrast, TCS had a higher purity almost to 100%. It is generally believed TCS, a type I RIP, is the active component. So the results make us consider why EOT has a better inhibitory effect on the growth of tumor cells than TCS. Whether the good anti-tumor activity of EOT is correlated with the cooperation of other proteins or any other factors. As was reported, cytotoxicity of TCS was dependent on its intracellular concentration, and variation of cytotoxicity in different cells might be related to the mechanisms affecting its internalization[17]. So there might be some active factors in EOT that promote internalization of TCS. Of course, the actual mechanism needs further researches to clarify.
It has been reported that TCS has strong effects on cytotoxicity and induction of apoptosis of gastric cancer cells, human choriocarcinoma cells and leukemia cells[8,18,19]. Also relative genes inducing apoptosis have been isolated with their mechanism elucidated[20]. In this study, we investigated apoptosis of HeLa cells induced by EOT. Experiments on morphology and biochemistry revealed typical apoptotic characteristics, such as microvilli disappearance, cell membrane bledding, apoptotic body and DNA ladder pattern. The results suggested that the anti-tumor effect of EOT on HeLa cells was related with the induction of apoptosis.
In conclusion, EOT has stronger anti-tumor effects and can induce apoptosis of HeLa cells. EOT would have a bright future in the treatment of tumors and further work may lead to relative anti-tumor agents to be used in clinic.
Edited by Wang XL and Xu CT Proofread by Xu FM
| 1. | Chan SH, Hung FS, Chan DS, Shaw PC. Trichosanthin interacts with acidic ribosomal proteins P0 and P1 and mitotic checkpoint protein MAD2B. Eur J Biochem. 2001;268:2107-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Lu PX, Jin YC. Trichosanthin in the treatment of hydatidiform mole. Clinical analysis of 52 cases. Chin Med J (Engl). 1990;103:183-185. [PubMed] |
| 3. | Chan SH, Shaw PC, Mulot SF, Xu LH, Chan WL, Tam SC, Wong KB. Engineering of a mini-trichosanthin that has lower antigenicity by deleting its C-terminal amino acid residues. Biochem Biophys Res Commun. 2000;270:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Wang JH, Nie HL, Huang H, Tam SC, Zheng YT. Independency of anti-HIV-1 activity from ribosome-inactivating activity of trichosanthin. Biochem Biophys Res Commun. 2003;302:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Wang JH, Nie HL, Tam SC, Huang H, Zheng YT. Anti-HIV-1 property of trichosanthin correlates with its ribosome inactivating activity. FEBS Lett. 2002;531:295-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Cai X, Yao G, Xu G, Yang C, Xu H, Lin Y, Yu J, Sun B. Identification of the amino acid residues in trichosanthin crucial for IgE response. Biochem Biophys Res Commun. 2002;297:510-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Chan WY, Huang H, Tam SC. Receptor-mediated endocytosis of trichosanthin in choriocarcinoma cells. Toxicology. 2003;186:191-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Tu SP, Jiang SH, Qiao MM, Cheng SD, Wang LF, Wu YL, Yuan YZ, Wu YX. Effect of trichosanthin on cytotoxicity and induction of apoptosis of multiple drugs resistance cells in gastric cancer. Shijie Huanren Xiaohua Zazhi. 2000;8:150-152. |
| 9. | Liu ZS, Tang SL, Ai ZL. Effects of hydroxyapatite nanoparticles on proliferation and apoptosis of human hepatoma BEL-7402 cells. World J Gastroenterol. 2003;9:1968-1971. [PubMed] |
| 10. | Kerr JF. History of the events leading to the formulation of the apoptosis concept. Toxicology. 2002;181-182:471-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 169] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Levy RR, Cordonier H, Czyba JC, Guerin JF. Apoptosis in preimplantation mammalian embryo and genetics. Ital J Anat Embryol. 2001;106:101-108. [PubMed] |
| 12. | Thatte U, Bagadey S, Dahanukar S. Modulation of programmed cell death by medicinal plants. Cell Mol Biol (Noisy-le-grand). 2000;46:199-214. [PubMed] |
| 13. | Wang Q. Trichosanthin. 2nd ed. Beijing: Sci Pub. 2000;21-28. |
| 14. | Yang ZR, Li JC. [Establishment and biological characteristics of two murine hepatocarcinoma substrains with different lymphatic metastatic ability]. Shiyan Shengwu Xuebao. 2003;36:99-104. [PubMed] |
| 15. | Ru QH, Luo GA, Liao JJ, Liu Y. Capillary electrophoretic determination of apoptosis of HeLa cells induced by trichosanthin. J Chromatogr A. 2000;894:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Wang Q. Trichosanthin. 2nd ed. Beijing: Sci Pub. 2000;298-319. |
| 17. | Chan WL, Zheng YT, Huang H, Tam SC. Relationship between trichosanthin cytotoxicity and its intracellular concentration. Toxicology. 2002;177:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Zhang C, Gong Y, Ma H, An C, Chen D, Chen ZL. Reactive oxygen species involved in trichosanthin-induced apoptosis of human choriocarcinoma cells. Biochem J. 2001;355:653-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Kong M, Ke YB, Zhou MY, Ke XY, Lu B, Nie HL. [Study on Trichosanthin induced apoptosis of leukemia K562 cells]. Shiyan Shengwu Xuebao. 1998;31:233-243. [PubMed] |
| 20. | Li XY, Wang GQ, Ge HL, Wang Y, Li NL, Zhu Q, Chen YL, Chou GY. [Isolation of a gene related to trichosanthin-induced apoptosis (GRETA)]. Shiyan Shengwu Xuebao. 2000;33:81-84. [PubMed] |