Published online Jul 15, 2004. doi: 10.3748/wjg.v10.i14.2063
Revised: December 28, 2003
Accepted: January 8, 2004
Published online: July 15, 2004
AIM: To evaluate the effects of animal milk containing fucosylated antigens on Helicobacter pylori (H pylori) binding to Lewis b antigen.
METHODS: A mammary gland expression vector containing human α1-3/4-fucosyltransferase cDNA sequences was constructed. Transient expression of human α1-3/4-fucosyltransferase cDNA in goat mammary cell and establishment of transgenic mice were performed. The adhesion inhibitory properties of milk samples were analyzed by using H pylori.
RESULTS: Goat milk samples were found to inhibit bacterial binding to Lewis b antigen. The highest inhibition was observed 42 h after injection of the plasmid. The binding activity of H pylori to Lewis b antigen reduced mostly, by 83%, however milk samples from transgenic mice did not inhibit H pylori binding to Lewis b antigen.
CONCLUSION: The use of “humanized” animal milk produced by the transgenic introduction of fucosylated antigen can perhaps provide an alternative therapy and preventive measure for H pylori infection.
-
Citation: Xu HT, Zhao YF, Lian ZX, Fan BL, Zhao ZH, Yu SY, Dai YP, Wang LL, Niu HL, Li N, Hammarström L, Borén T, Sjöström R. Effects of fucosylated milk of goat and mouse on
Helicobacter pylori binding to Lewis b antigen. World J Gastroenterol 2004; 10(14): 2063-2066 - URL: https://www.wjgnet.com/1007-9327/full/v10/i14/2063.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i14.2063
Helicobacter pylori (H pylori), a human specific gastric pathogen, was first isolated in 1983[1]. Twenty years of research has found that H pylori infection is one of the major causes of upper gastrointestinal tract diseases, such as chronic active gastritis and peptic ulcer disease[2-6]. In chronic active gastritis, gastric ulcer and gastroduodenal ulcer, the incidences of H pylori infection are 71%-94%, 72%-100% and 73%-100% respectively. In addition, H pylori infection has been linked with the development of gastric adenocarcinoma and mucosa-associated lymphoid tissue (MALT)[7-13]. It has been defined as a Class I carcinogen by WHO[14,15].
H pylori colonize human gastric mucosa by adhering both to the mucous epithelial cells and to the mucus layer[16]. Specific receptor structures in combination with the unique tissue-specific distribution of receptors can restrict microbial colonization to a limited number of hosts, tissues and cell lineages[17]. H pylori can bind tightly to epithelial cells using various bacterial surface components[18-20]. The best characterized adhesin, BabA, is a 78-kD outer-membrane protein that binds to the fucosylated Lewis b (Leb) blood group antigen[21]. Accumulating evidence in animal models suggests that BabA is relevant to H pylori-associated diseases[22]. Leb antigen is one of the most important receptors, governing adhesion of H pylori to gastric mucosa. Boren et al[17] found that the fucosylated Lewis blood group antigens Leb and H-1 were the carbohydrate structures that specifically mediated the adherence of H pylori to human gastric epithelial cells in situ.
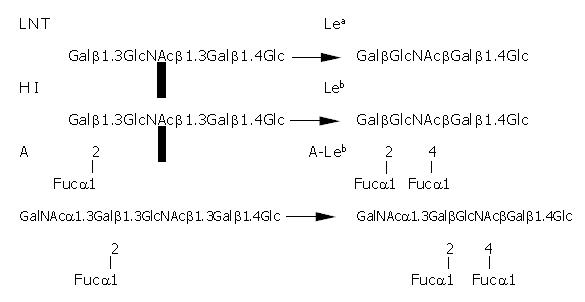
Leb antigen is a human blood group antigen. In human cells, the synthesis of Lewis antigens is regulated by a series of glycosyltransferases that act sequentially upon a precursor molecule. The fucosyltransferases are responsible for the final step in this process. Their function is to add a fucose residue to precursor molecules to form human blood antigens, such as Lea, Leb and H1 antigen (Figure 1). Addition of fucose to the terminal galactose residue of the lacto series core chain oligosaccharide results in the H1 antigen. Leb antigen is formed by the addition of a “branched” fucose residue to H1-antigen, catalyzed by α1-3/4-fucosyltransferase. The fucosylated blood group antigens, typically found on erythrocytes, are also expressed on the gastro-intestinal epithelium. Leb is the dominant fucosylated blood group antigen expressed on the gastric surface mucous cells in the gastric epithelial lining. The fucosylated blood group antigens are also present in the mucins of the gastric mucus layer and, in addition, as natural “scavengers” or clearance factors in secretions such as saliva, tears, and human milk.
H pylori infection is one of the most common infections in humans. Epidemiological data show that it affects about half of the human population. Without specific therapy, H pylori infection can persist for decades or even the host’s lifetime. But only about 15% of H pylori-infected individuals actually have H pylori-associated diseases. It is likely to be associated with other additional factors such as genetic predisposition, age of infection, and the genotype of infective strain. The prevalence varies greatly among countries and among population groups within the same country. The overall prevalence of H pylori infection is strongly correlated with socioeconomic conditions. Eighty-five percent of H pylori can be eradicated by combination therapy in clinic, however, using antibiotics for several weeks may bring about other problems such as bacterial resistance. So many researchers are looking for other methods to prevent H pylori infection, such as preventing H pylori binding to or colonizing the gastric mucosa.
If we can add some H pylori specific receptor, for example, Leb blood antigen or its analog to food, then H pylori binding to the human gastric mucosa may be prevented or reduced, and the bacteria will be excreted by the alimentary tract or destroyed by human antibody. Flak et al reported that the α1-3/4-fucosyltransferase was expressed in the gastric mucosa of mice and that H pylori could bind to the mice gastric mucosa. At present, there are no reports of α1-3/4-fucosyltransferase being expressed in animal galactophore. Therefore, our aim was first to introduce human α1-3/4-fucosyltransferase into animals and get them expressed in the animal mammary gland and thus produce Leb antigen in milk. This kind of milk does not only have nutritional value, it is also a natural source of lectin, a molecule that can block H pylori binding to the human gastric mucosa, and therefore prevent H pylori infection and reduce the severity of the infectious process. In this way, people can prevent H pylori infection by drinking this kind of milk daily.
This paper describes the transient expression of human α1-3/4-fucosyltransferase gene in goat mammary gland and the establishment of transgenic mouse model. A new test to prevent and cure H pylori infection and gastrointestinal diseases associated with H pylori infection is put forward in our study.
Kunming white mice were purchased from Beijing Laboratory Animal Research Center. Laoshan goats were provided by Beijing Sangao Corporation.
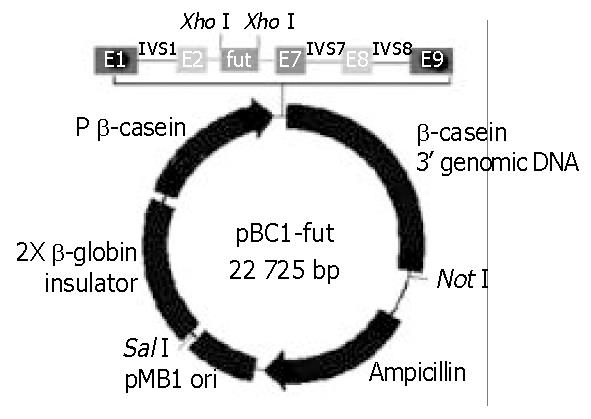
A 1115-bp α1-3/4-fucosyltransferase cDNA encompassing the entire 1086-nt coding region specifying the 361-AA transmembrane glycoprotein, containing an upstream Kozak consensus sequence and XhoI site, a downstream XhoI site, was generated by PCR from a Fut/pCDM8 plasmid. The PCR product was then purified and digested by XhoI, and the digestion product was subcloned into pBC1 vector, which had been treated previously with XhoI, and transformed into E. coli DH5α. pBC1 is a specific milk expression vector which contains the goat β-casein promoter and other proprietary DNA sequences. The positive transfected clone containing the properly oriented α1-3/4-fucosyltransferase cDNA was screened by colony PCR. The expression vector, named pBC1-fut, allowed the α1-3/4-fucosyltransferase cDNA to be placed in its downstream of the β-casein promoter (Figure 2).
pBC1-fut was purified using Qiagen Plasmid Maxi kit. About 1 mg pBC1-fut was injected into a lactating goat’s right and left mammary glands from the goat glandular duct. Milk samples at different times were then collected over a 100-h (4 d) period from both right (R) and left (L) udders. Then, milk samples were analyzed in a dilution series (25-, 50-, 100- and 200-fold) for adhesion inhibition properties.
The 16-kb DNA fragment inserted was isolated by agarose gel electrophoresis and recovered by electro-elution. To remove any contamination, products were spot dialyzed against 40 mL TE (10 mmol/L Tris, 0.1 mmol/L EDTA, pH7.4) for 30 min (VSWP02500 membrane, Millipore). Purified DNAs were diluted to 2-3 ng/μL in TE buffer and microinjected into the pronuclei of fertilized eggs of Kunming white mice.
Genomic DNAs were isolated from the tails of transgenic mice using a standard method. A pair of primers was designed to screen for transgenic mice: upper primer: 5’- GATTGACAAGTAATACGCTGTTTCCTC-3’ and downstream primer: 5’-CATCAGAAGTTAAACAGCACAGTTAG-3’. PCR reactions using genomic DNAs as template were performed under the following condition: 30 cycles of 94 °C for 1 min, 58 °C for 1 min, and 74 °C for 1 min. After PCR screening, transgenic mice were confirmed by Southern hybridization. The probe was created by 32P labeling α1-3/4-fucosyltransferase cDNA. Genomic DNA from transgenic mice and negative mouse as well as expression vector DNA were digested by BamHI. Copies of the transgene were estimated by comparing the band density of the vector control with that in transgenic mice. Hybridizations were at 65 °C in Church (10 g/L BSA, 70 g/L SDS, 1 mmol/L EDTA, 0.5 mol/L sodium phosphate, pH7.2). Final washes were in 2 × SSC, 0. 5 × SDS at 65 °C. Signal from the membrane was detected using a Phosphor Screen (Molecular Dynamics, US).
Goat milk was collected at different time points for 100 h from both right (R) and left (L) udders. The transgenic milk was collected at the 7 th day of lactation. The milk was centrifuged at 18000 r/min for 1 h at 4 °C. The fat on the surface was removed and the clear part of the supernatant was put in a new tube and used as the sample. Leb antigen was labeled with 125I by the chloramine T method. Milk samples were analyzed in dilution series (25-, 50-, 100- and 200-fold). The samples were mixed with an H pylori strain (CCUG17875), which bound bind Leb antigen efficiently, on a cradle for 17 h at room temperature. After this period, 125I radioactivity in bacterial pellet was measured with a gamma counter.
After electrophoresis on 80 g/L SDS-PAGE, proteins were transferred to a nitrocellulose extra blotting membrane (Sartorius, Germany). Leb monoclonal antibodies (Immucor, GA) and HRP-conjugated goat anti-rabbit-IgG (Cappel Laboratories, US) were used to detect Leb antigen.
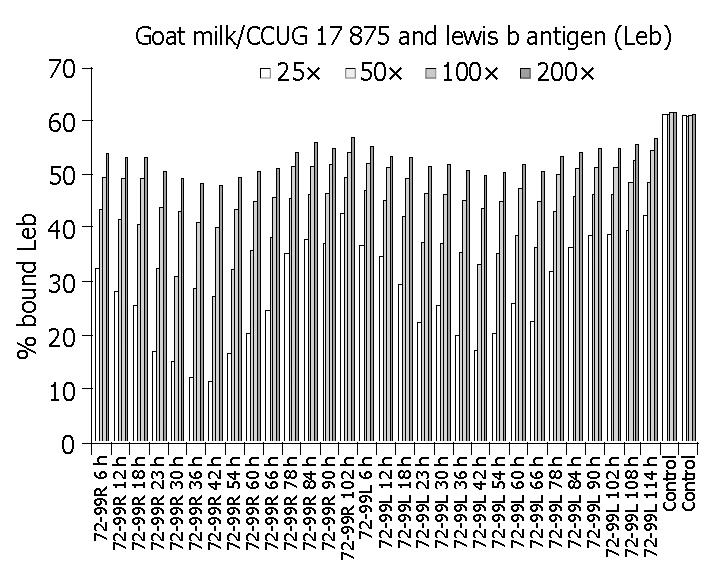
As shown in Table 1 and Figure 3, milk samples from experimental goats inhibited H pylori binding to Leb antigen, and the time of the highest inhibition efficacy was at 42 h after DNA immunization. Furthermore, milk collected from both right (R) and left (L) udders had inhibitory effect on H pylori binding to Leb. Unimmunized goat (the negative controls) did not inhibit bacterial binding. The binding activity of H pylori to Leb antigen reduced mostly, 83% in the 25-fold diluted milk samples.
| Time | Bind/Free Leb* (%) | Sample | Bind/Free Leb* (%) | ||||||
| 25 × | 50 × | 100 × | 200 × | 25 × | 50 × | 100 × | 200 × | ||
| R 6 h | 32.5 | 43.4 | 49.6 | 53.9 | L 6 h | 36.9 | 47.1 | 52.1 | 55.1 |
| R 12h | 28.2 | 41.7 | 49.4 | 53.0 | L 12 h | 34.7 | 45.3 | 51.3 | 53.4 |
| R 18 h | 25.5 | 40.5 | 49.4 | 53.1 | L 18 h | 29.4 | 42.3 | 49.2 | 53.2 |
| R 23 h | 16.8 | 32.3 | 43.9 | 50.6 | L 23 h | 22.1 | 37.3 | 46.5 | 51.7 |
| R 30 h | 15.0 | 31.0 | 43.1 | 49.4 | L 30 h | 25.6 | 37.1 | 46.3 | 51.9 |
| R 36 h | 11.9 | 28.7 | 41.2 | 48.3 | L 36 h | 20.0 | 35.4 | 45.1 | 50.8 |
| R 42 h | 11.2 | 27.0 | 40.1 | 48.0 | L 42 h | 17.2 | 33.3 | 43.6 | 49.7 |
| R 54 h | 16.6 | 32.3 | 43.4 | 49.5 | L 54 h | 20.3 | 35.1 | 44.9 | 50.4 |
| R 60 h | 20.2 | 35.9 | 45.1 | 50.5 | L 60 h | 25.9 | 38.7 | 47.6 | 51.7 |
| R 66 h | 24.6 | 38.3 | 45.8 | 51.0 | L 66 h | 22.6 | 36.2 | 44.9 | 50.6 |
| R 78 h | 35.2 | 45.6 | 51.6 | 54.2 | L 78 h | 31.9 | 43.1 | 50.2 | 53.3 |
| R 84 h | 37.8 | 46.2 | 51.7 | 56.0 | L 84 h | 36.3 | 45.9 | 51.1 | 54.0 |
| R 90 h | 37.1 | 46.5 | 51.9 | 54.8 | L 90 h | 38.5 | 46.3 | 51.3 | 54.8 |
| R 102 h | 42.6 | 49.4 | 54.1 | 57.1 | L 102 h | 38.8 | 46.3 | 51.3 | 55.0 |
| Control 1 | 61.2 | 61.2 | 61.5 | 61.5 | Control 2 | 61.0 | 60.8 | 61.1 | 61.2 |
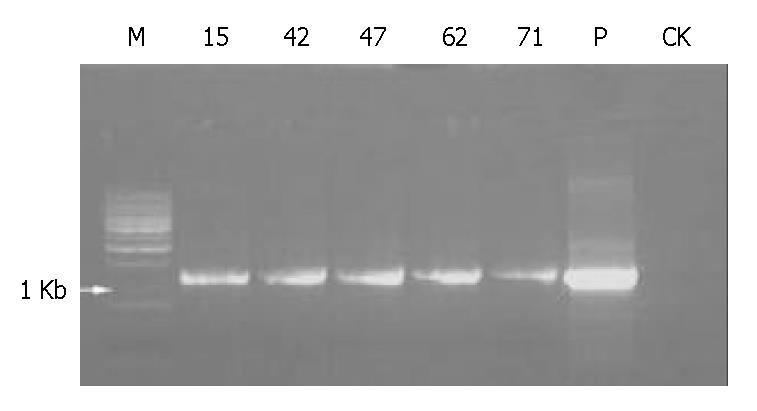
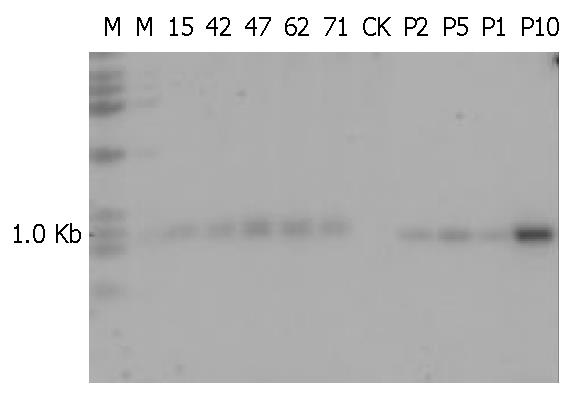
Five of 84 mice including 2 males and 3 females were identified as being transgenic mice by PCR (Figure 4). The serial numbers of the positive mice were 15, 42, 47, 63 and 71. Efficiency of microinjection was about 6%, within the usual range of 5%-20%. These transgenic mice were confirmed by using human α1-3/4-fucosyltransferase cDNA as a probe in Southern blotting (Figure 5). Transgene copy numbers were also determined by Southern blotting. We analyzed three female’s milk for the blocking effect on binding of H pylori to Leb antigen, but no inhibitory activity was detected by our experimental system.
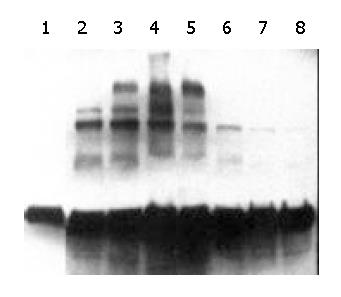
We performed Western blotting and stained the membranes of the goat and transgenic mice milk protein with Leb monoclonal antibody. The goat milk sample blot showed a beautiful, time-dependent induction of the blood group antigens secreted into milk (Figure 6). The band densities at different time points were consistent with the results of the transient expression described above. The band density was strongest at the 42 h after DNA immunization. But the transgenic mice milk did not give a positive signal, suggesting that this milk did not contain Leb antigen or its analog.
In our experiment, goat milk could block H pylori binding to Leb antigen, which is one of the most important receptors governing adhesion of H pylori to gastric mucosa. The activity of H pylori binding to Leb antigen reduced as much as 83% in some samples. The result showed that some milk proteins might be fucosylated and structurally similar to human Leb blood group antigen, so they were able to bind the bacteria. Goat milk fucosylated protein can bind H pylori in vitro. So “humanized” goat milk, in which human α1-3/4-fucosyltransferase has been introduced into goat mammary gland, may be an alternative therapy and a prevention method for H pylori infection.
Unfortunately, the transgenic mice milk collected did not block H pylori binding to Leb antigen. The reasons might be as follows: (1) Level of α1-3/4-fucosyltransferase gene expression in mammary gland of mice was very low. Thus the quantity of Leb antigen in the milk was so low that the milk could not effectively block H pylori binding to Leb antigen. One way to increase the expression of α1-3/4-fucosyltransferase would be to introduce the complete genomic sequence of the α1-3/4-fucosyltransferase gene to mammary gland of mice. (2) There is no precursor of Leb antigen in mice milk, so, even if α1-3/4-fucosyltransferase was expressed in mouse galactophore, the transgenic glycosylation patterns that were generated by the activity of α1-3/4-fucosyltransferase did not form epitopes that were recognized by the H pylori Leb-binding adhesions. Therefore the milk could not block H pylori binding to Leb antigen. Since other results have shown that α1-3/4-fucosyltransferase expression in the gastric mucosa of mice can block the binding of H pylori to mouse gastric mucosa, it is possible that there might be differences between the carbohydrate core chains of the Leb antigen in milk glands and gastric mucosa in mice.
Although the results for the transgenic mice were not positive, the successful introduction of α1-3/4-fucosyltransferase cDNA into goat mammary cell and expression of Leb antigen analog were an important finding. The “humanized” milk by the transgenic introduction of fucosylated antigens can be an alternative therapy and a prevention method for H pylori infection.
Edited by Chen WW and Zhu LH Proofread by Xu FM
| 1. | Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273-1275. [PubMed] |
| 2. | Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1229] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 3. | Hansson LE, Nyrén O, Hsing AW, Bergström R, Josefsson S, Chow WH, Fraumeni JF, Adami HO. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N Engl J Med. 1996;335:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 430] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Dooley CP, Cohen H, Fitzgibbons PL, Bauer M, Appleman MD, Perez-Perez GI, Blaser MJ. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989;321:1562-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 499] [Article Influence: 13.9] [Reference Citation Analysis (2)] |
| 5. | Eck M, Schmausser B, Haas R, Greiner A, Czub S, Müller-Hermelink HK. MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology. 1997;112:1482-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 138] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Wang RT, Wang T, Chen K, Wang JY, Zhang JP, Lin SR, Zhu YM, Zhang WM, Cao YX, Zhu CW. Helicobacter pylori infection and gastric cancer: evidence from a retrospective cohort study and nested case-control study in China. World J Gastroenterol. 2002;8:1103-1107. [PubMed] |
| 7. | Eid R, Moss SF. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2002;346:65-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Parsonnet J, Isaacson PG. Bacterial infection and MALT lymphoma. N Engl J Med. 2004;350:213-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991;302:1302-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 924] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 10. | Fox JG. Review article: Helicobacter species and in vivo models of gastrointestinal cancer. Aliment Pharmacol Ther. 1998;12 Suppl 1:37-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3182] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 12. | Wotherspoon AC. Helicobacter pylori infection and gastric lymphoma. Br Med Bull. 1998;54:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Wotherspoon AC. Gastric lymphoma of mucosa-associated lymphoid tissue and Helicobacter pylori. Annu Rev Med. 1998;49:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994.. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [PubMed] |
| 15. | Bayerdörffer E, Neubauer A, Rudolph B, Thiede C, Lehn N, Eidt S, Stolte M. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet. 1995;345:1591-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 595] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 16. | Karlsson KA. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem. 1989;58:309-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 505] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 17. | Borén T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 804] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 18. | Mahdavi J, Sondén B, Hurtig M, Olfat FO, Forsberg L, Roche N, Angstrom J, Larsson T, Teneberg S, Karlsson KA. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 665] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 19. | Marshall B. Helicobacter pylori: 20 years on. Clin Med. 2002;2:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Odenbreit S, Faller G, Haas R. Role of the alpAB proteins and lipopolysaccharide in adhesion of Helicobacter pylori to human gastric tissue. Int J Med Microbiol. 2002;292:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Lindén S, Nordman H, Hedenbro J, Hurtig M, Borén T, Carlstedt I. Strain- and blood group-dependent binding of Helicobacter pylori to human gastric MUC5AC glycoforms. Gastroenterology. 2002;123:1923-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Guruge JL, Falk PG, Lorenz RG, Dans M, Wirth HP, Blaser MJ, Berg DE, Gordon JI. Epithelial attachment alters the outcome of Helicobacter pylori infection. Proc Natl Acad Sci USA. 1998;95:3925-3930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 205] [Article Influence: 7.6] [Reference Citation Analysis (0)] |