Published online Jun 15, 2004. doi: 10.3748/wjg.v10.i12.1830
Revised: October 15, 2003
Accepted: October 22, 2003
Published online: June 15, 2004
AIM: To investigate the mechanisms of tegaserod, a partial 5-HT4 agonist, in reducing visceral sensitivity by observing Fos, substance P (SP) and calcitonin gene-related peptide (CGRP) expression in the lumbarsacral spinal cord induced by colonic inflammation in rats.
METHODS: Twenty-four male rats with colonic inflammation induced by intraluminal instillation of trinitrobenzenesulfonic acid (TNBS) were divided into 3 groups. Treatment group 1: intra-gastric administration of tegaserod, 2 mg/kg·d; Treatment group 2: intra-gastric administration of tegaserod, 1 mg/kg·d; Control group: intra-gastric administration of saline, 2.0 mL/d. After 7 d of intra-gastric administration, lumbarsacral spinal cord was removed and processed for Fos, SP and CGRP immunohistochemistry.
RESULTS: In rats of the control group, the majority of Fos labeled neurons was localized in deeper laminae of the lumbarsacral spinal cord (L5-S1). SP and CGRP were primarily expressed in the superficial laminae of the spinal cord after TNBS injection. Intra-gastric administration of tegaserod (2 mg/kg·d) resulted in a significant decrease of Fos labeled neurons (22.0 ± 7.7) and SP density (12.5 ± 1.4) in the dorsal horn in the lumbarsacral spinal cord compared to those of the control group (62.2 ± 18.9, 35.9 ± 8.9, P < 0.05). However, CGRP content in dorsal horn did not significantly reduce in rats of treatment group 1 (1.2 ± 1.1) compared to that of the control group (2.8 ± 2.4, P > 0.05). Neither Fos expression nor SP or CGRP density in the dorsal horn significantly declined in rats of treatment group 2 compared to those of the control group (P > 0.05).
CONCLUSION: Tegaserod can significantly reduce Fos labeled neurons in the lumbarsacral spinal cord induced by colonic inflammation. Tegaserod may reduce visceral sensitivity by inhibiting SP expression in the dorsal horn of spinal cord.
- Citation: Sun YN, Luo JY. Effects of tegaserod on Fos, substance P and calcitonin gene-related peptide expression induced by colon inflammation in lumbarsacral spinal cord. World J Gastroenterol 2004; 10(12): 1830-1833
- URL: https://www.wjgnet.com/1007-9327/full/v10/i12/1830.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i12.1830
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder. Its typical feature is recurrent abdominal discomfort or pain associated with disordered defecation. Studies have shown that visceral sensitivity plays an important role in the pathophysiology of IBS[1]. Tegaserod, a partial 5-HT4 agonist, acts selectively on 5-HT4 receptors in the gut. Tegaserod can relieve symptoms in IBS patients with abdominal discomfort or pain and significantly decrease visceral sensitivity[2]. The objectives of the present study were to identify regions of the Fos protein, substance P (SP) and calcitonin gene-related peptide (CGRP) expression in the lumbarsacral spinal cord in response to colonic inflammation induced by intraluminal instillation of TNBS, and to compare the effects of tegaserod on the spinal Fos, SP and CGRP expression induced in the same animal model.
Twenty-four adult male Sprague-Dawley rats (The Fourth Military Medical University) weighing 220-250 g were used in this study. The animals were housed in a quiet room with a constant ambient temperature of 20 °C with free access to rat chow and water.
After 24 h fast, TNBS (100 mg/kg in 300 mL/L ethanol) was administered intrarectally through a silicone rubber catheter introduced 7 cm into the anus under light diethyl-ether anesthesia. To keep TNBS in the colon for a longer time and to avoid leakage, the catheter was slowly withdrawn and the tail of the rat was kept elevating for 8-10 min.
Tegaserod (20 mg, Beijing Novartis Pharmacy Company) was dissolved in 100 mL distilled water. After one day of TNBS instillation, the rats were randomly divided into 3 groups. Each group had eight rats. Treatment group 1: intra-gastric administration of tegaserod 2 mg/kg; Treatmemt group 2: intra-gastric administration of tegaserod 1 mg/kg; Control group: intra-gastric administration of saline 2.0 mL. Tegaserod or saline was administered once daily (10:00AM) for consecutive 7 d after TNBS instillation.
One day after the last time of tegaserod or saline administration, the rats were deeply anaesthetized with pentobarbital Na (80 mg/kg, i.p.) and perfused with 100 mL saline followed by 500 mL fixative of 40 g/L paraformaldehyde in 0.1 mol/L phosphate buffer (PH7.4). L3-S2 segments of the spinal cord were removed, post-fixed in the same fixative at 4 °C for 2-4 h and then cryoprotected in 200 g/L sucrose overnight. Serial frozen sections of 40-μm thickness cut on a Leitz cryocut. Sections were collected in 0.01 mol/L PBS for immunohistochemistry.
The spinal cord sections were stained for Fos, SP and CGRP using avidin-biotin-peroxidase complex (ABC) method. Free floating tissue sections were treated in 800 mL/L methanol containing 30 mL/L H2O2 to block endogenous peroxidase acitvity for 30 min at room temperature. They were treated with 0.01 mol/L PBS containing 1 g/L Triton ×100 for 20 min at room temperature. The sections were then incubated with a polyclonal rabbit anti-Fos serum (1:3 000, Santa Cruz), polyclonal rabbit anti-SP serum (1:500, Boster) and polyclonal rabbit anti-CGRP serum (1:500, Boster) respectively for 48 h at 4 °C. After the step, the sections were incubated with biotinylated goat anti-rabbit IgG (1:500, Sigma), and subsequently with the ABC complex (1:500, Sigma) at room temperature for 2 h each. The antigen-antibody reaction sites were visualized by incubation with glucose oxidase-DAB-nickel method for 15-30 min at room temperature. The sections were rinsed in 0.01 mol/L PBS for 3×10 min between transition of these steps. Finally, the sections were mounted onto gelatin-coated slides, dried, dehydrated, cleared and coverslipped.
The number of neuronal nuclear profiles expressing Fos was analyzed with computer assisted QUIC menu system. Precise regional localization was done by outlining specific regions and then particles (stained nuclei) were counted in the outlined regions. The number of profiles counted in five sections was averaged for each animal, and comparisons were made between the groups[3].
The density of SP and CGRP staining in the dorsal horn of spinal cord was also measured in specific outlined regions using computer assisted QUIC menu system. The ratio of the area of stained SP or CGRP to the area of the outlined regions was presented as the density of SP or CGRP. The averages were obtained for density measurements from five randomly selected spinal cord sections for each animal, and comparisons were made between treatment and control groups[3].
Pictures were taken under BX-60 microscopy with the support of IM50 software. All results are expressed as mean ± SD. An unpaired t-test was used to compare Fos-IR (immunoreactive) cell count data, SP and CGRP density between the groups. A difference was accepted as significant if the probability was less than 5% (P < 0.05).
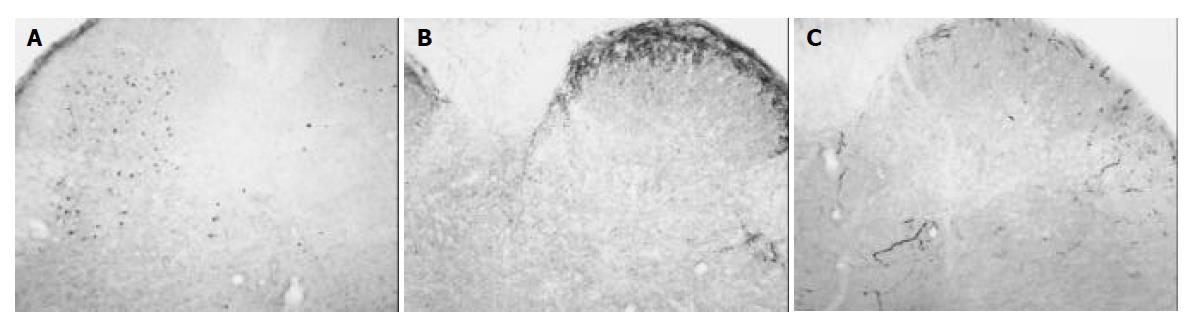
In animals of the control group, Fos protein was expressed bilaterally in the nuclei of cells of the dorsal horn in L5, L6 and S1 of lumbarsacral spinal cord. The nuclei of Fos-IR neurons were black and there was no cytoplasmic staining. Among 62.2 ± 18.9 neurons exhibited Fos-IR, most of them were distributed in the deeper laminae (III-IV, V-VI) of the dorsal horn. Only few Fos-IR neurons were sparsely distributed in the superficial laminae (I-II) of the dorsal horn (Figure 1A).
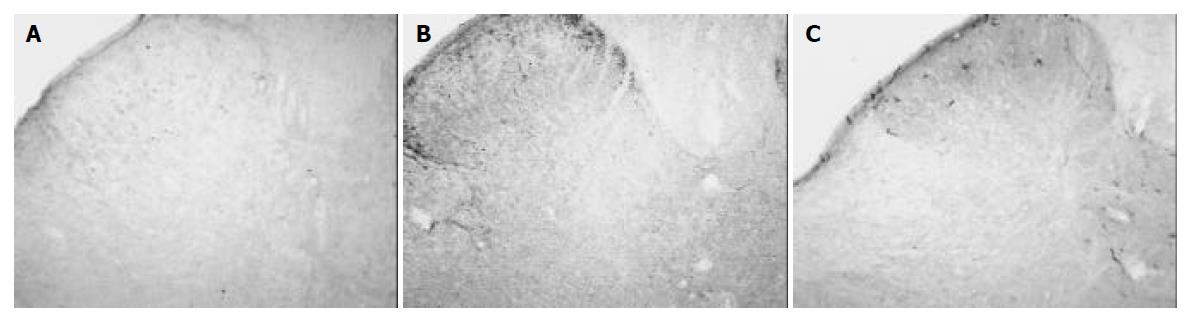
After intra-gastric administration of tegaserod 1 mg/kg·d for 7 d, Fos-IR cells in the dorsal horn decreased to 42.6 ± 7.5, but was not significantly different with that of the control group (62.2 ± 18.9, P > 0.05). After intra-gastric administration of tegaserod 2 mg/kg·d for seven days, Fos-IR neurons in the dorsal horn decreased to 22.0 ± 7.7, a significant difference (P < 0.05) (Table 1, Figure 2A).
In rats of the control group, SP was expressed bilaterally in the superficial laminae (I-II) in the dorsal horn. The density of SP was 35.9 ± 8.9. After 7 d of intra-gastric injection of tegaserod 1mg/kg·d, the density of SP in the superficial laminae did not significantly reduce (33.3 ± 3.4, P > 0.05). However, after intra-gastric administration of tegaserod 2 mg/kg·d for 7 d, the density of SP decreased to 12.5 ± 1.4, a significant difference compared to that of the control group (P < 0.05) (Table 1, Figures 1B, 2B).
In animals of the control group, most CGRP were expressed in the superficial laminae of the dorsal horn. A few CGRP were distributed in the deeper laminae. The density of CGRP in rats of the control group was 2.8 ± 2.4. The density of CGRP did not significantly decrease in rats treated with tegaserod 1 mg/kg·d for 7 d (2.2 ± 2.3, P > 0.05). After treatment with tegaserod 2 mg/kg·d for 7 d, the density of CGRP decreased 56.7% compared to that of the control group (1.2 ± 1.1), but there was no significant difference (P > 0.05) (Table 1, Figures 1C, 2C).
Irritable bowel syndrome is a common functional gastrointestinal disorder. Its typical feature is recurrent abdominal discomfort or pain associated with disordered defecation or a change in bowel habit. Routine investigation revealed no evidence of structural or biochemical abnormality. There is evidence that patients with IBS showed a lower threshold for discomfort and/or pain, increased sensitivity to stimuli, or altered viscerosomatic referral[4]. Therefore, visceral hypersensitivity could play an important role in the pathophysiology of IBS[1].
Tegaserod is a partial 5-HT4 agonist. The absence of blood-brain barrier permeation makes it selectively bind to 5-HT4 receptors in the gastrointestinal tract. Tegaserod has been shown to modulate both gastrointestinal motility and visceral sensitivity[2]. Studies demonstrated that tegaserod produced rapid and sustained improvements of the abdominal pain/ discomfort and constipation in IBS patients, especially in female IBS patients[5,6]. These improvements were seen within the first week, and maintained throughout the treatment period. A randomized, double-blind, placebo-controlled study showed that tegaserod reduced the sensitivity to rectal distension in healthy subjects[7].
Fos, one of the inducible transcription factors, serves as a quantifiable marker to identify neuronal populations activated by noxious somatic and visceral stimulations. Noxious stimulation of hollow viscera induced a specific pattern of Fos expression in rat spinal cord that reflected the intensity of the stimulation[8]. Recently, the anti-nociceptive effects of some drugs have been studied in animal models of persistent visceral pain using Fos expression as an independent measure. Lu reported that baclofen, a selective GABAB receptor agonist, specifically reduced Fos expression in the superficial dorsal horn of spinal cord induced by mustard oil irritation of the colon[3]. A recent study has demonstrated that opiate receptor agonists, especially morphine, attenuate pain related behavior and Fos expression in rat spinal cord neurons following noxious visceral stimulation[9]. Thus, localization of Fos expression can be a useful tool to examine the effectiveness of different analgesic drugs after nociceptive stimulation.
In rats of the control group, Fos protein expression became bilaterally evident as nuclear staining in cells in L5, L6 and S1 of lumbarsacral spinal cord. In the rats, the descending colon was innervated by sensory afferent fibers in the pelvic nerve projecting bilaterally to the lumbarsacral (L6-S2) spinal cord[10]. The result was consistent with the viscerotopic organization of the afferent fibers that innervate the descending colon. Most of the Fos-IR neurons were distributed in the deeper laminae (III-IV, V-VI) of the dorsal horn. Few Fos expressed in the superficial laminae (I-II) of the dorsal horn. Imbe demonstrated that Fos-IR cells had the tendency to spread from the superficial laminae to the deeper laminae when deep tissue inflammation became persistent[11]. From the segment and laminae distribution of Fos-IR neurons, we conclude that neurons in the dorsal horn of lumbarsacral spinal cord are activated via sensory afferent fibers in the pelvic nerve by noxious stimulation induced by persistent colonic inflammation. Therefore, tegaserod can dose-dependently prevent sensory neurons in the dorsal horn from being activated by colonic inflammation.
Substance P was released from primary sensory nociceptive neurons in response to noxious stimuli. Up to 80% of dorsal root ganglion (DRG) neurons supplying viscera contained SP, suggesting the importance of SP in transmission of signals from the viscera to the spinal cord[12]. Study of Kishimoto demonstrated that high concentrations of SP were significantly related to abdominal pain[13]. SP could play a pivotal role at the spinal cord level in postsynaptic sensitization, particularly during and after gut inflammation[14]. In the spinal cord, SP releasing from the primary afferents could activate the dorsal horn neurons by inducing an influx of Ca2+ throughout voltage-gated Ca2+ channels[15].The results of this study showed that the density of SP in the dorsal horn decreased significantly compared to the control group after treatment with tegaserod 2 mg/kg·d for 7 d . Therefore, tegaserod can inhibit SP expression in the dorsal horn of the spinal cord.
CGRP is one of the major neuropeptides expressed in the spinal cord sensory processing region and is widely distributed in the nervous system, particularly in sensory neurons. CGRP, which is generally colocalized with SP in the primary afferent nociceptors, is likely to be involved in pain transmission. CGRP may produce a direct nociceptive effect. CGRP might also enhance SP-induced nociceptive sensation by facilitating the release of SP in the spinal dorsal horn[16]. Compared with that of the control group, the density of CGRP decreased 21.4% in treatment group 1 and 57.1% in treatment group 2, showing an insignificant difference (P > 0.05). The results suggest that tegaserod may not act on CGRP expression to reduce visceral nociceptive transmission. However, whether a much larger dose of tegaserod can significant decrease CGRP expression in the spinal cord still needs more studies.
The results of the present study showed that tegaserod could significantly decrease Fos and SP expression in the dorsal horn of the spinal cord. Tegaserod can exert antinociceptive effect. This effect is related to SP expression inhibition in the spinal cord. The study of Schikowski showed that in decerebrated cats the static discharge rate of the afferents evoked by rectal distension decreased significantly after intravenous administration of tegaserod. They proposed that 5-HT4 receptor activation had an inhibitory effect on intramural mechanoreceptors in cat rectum[17]. Today there is considerable evidence that serotonin influences nociceptive reflexes at different anatomic levels. For example, intrathecal and iontophoretic applications of serotonin to the dorsal horn produced pain relief, and serotonin antagonists produced hyperalgesia[18]. Ghelardini found that BIMU1 and BIMU8, two 5-HT4 agonists, exerted an antinociceptive effect by centrally potentiating endogenous cholinergic activity[19]. Since there are serotonin-SP and serotonin-Ach neurotransmitter links, we conclude that tegaserod, acting on 5-HT4 receptors in the gut, produces an antinociceptive effect by inhibiting the expression of SP in spinal dorsal horn.
Edited by Zhao M and Wang XL Proofread by Xu FM
| 1. | Bueno L, Fioramonti J, Delvaux M, Frexinos J. Mediators and pharmacology of visceral sensitivity: from basic to clinical investigations. Gastroenterology. 1997;112:1714-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 171] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Lacy BE, Yu S. Tegaserod: a new 5-HT4 agonist. J Clin Gastroenterol. 2002;34:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Lu Y, Westlund KN. Effects of baclofen on colon inflammation-induced Fos, CGRP and SP expression in spinal cord and brainstem. Brain Res. 2001;889:118-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 679] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 5. | Müller-Lissner SA, Fumagalli I, Bardhan KD, Pace F, Pecher E, Nault B, Rüegg P. Tegaserod, a 5-HT(4) receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther. 2001;15:1655-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 336] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | Novick J, Miner P, Krause R, Glebas K, Bliesath H, Ligozio G, Rüegg P, Lefkowitz M. A randomized, double-blind, placebo-controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2002;16:1877-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 209] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Coffin B, Farmachidi JP, Rueegg P, Bastie A, Bouhassira D. Tegaserod, a 5-HT4 receptor partial agonist, decreases sensitivity to rectal distension in healthy subjects. Aliment Pharmacol Ther. 2003;17:577-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Traub RJ, Pechman P, Iadarola MJ, Gebhart GF. Fos-like proteins in the lumbosacral spinal cord following noxious and non-noxious colorectal distention in the rat. Pain. 1992;49:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Traub RJ, Stitt S, Gebhart GF. Attenuation of c-Fos expression in the rat lumbosacral spinal cord by morphine or tramadol following noxious colorectal distention. Brain Res. 1995;701:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Traub RJ, Murphy A. Colonic inflammation induces fos expression in the thoracolumbar spinal cord increasing activity in the spinoparabrachial pathway. Pain. 2002;95:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Imbe H, Iwata K, Zhou QQ, Zou S, Dubner R, Ren K. Orofacial deep and cutaneous tissue inflammation and trigeminal neuronal activation. Implications for persistent temporomandibular pain. Cells Tissues Organs. 2001;169:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Palecek J, Paleckova V, Willis WD. Postsynaptic dorsal column neurons express NK1 receptors following colon inflammation. Neuroscience. 2003;116:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Kishimoto S, Kobayash H, Machino H. High concentrations of substance P as a possible transmission of abdominal pain in rats with chemical induced ulcerative colitis. Biomed Res. 1994;15:133-140. |
| 14. | Bueno L, Fioramonti J. Effects of inflammatory mediators on gut sensitivity. Can J Gastroenterol. 1999;13:42A-46A. [PubMed] |
| 15. | Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1458] [Cited by in RCA: 1301] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 16. | Friese N, Diop L, Chevalier E, Angel F, Rivière PJ, Dahl SG. Involvement of prostaglandins and CGRP-dependent sensory afferents in peritoneal irritation-induced visceral pain. Regul Pept. 1997;70:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Schikowski A, Thewissen M, Mathis C, Ross HG, Enck P. Serotonin type-4 receptors modulate the sensitivity of intramural mechanoreceptive afferents of the cat rectum. Neurogastroenterol Motil. 2002;14:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Gyermek L. Pharmacology of serotonin as related to anesthesia. J Clin Anesth. 1996;8:402-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Ghelardini C, Galeotti N, Casamenti F, Malmberg-Aiello P, Pepeu G, Gualtieri F, Bartolini A. Central cholinergic antinociception induced by 5HT4 agonists: BIMU 1 and BIMU 8. Life Sci. 1996;58:2297-2309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |