Published online May 15, 2004. doi: 10.3748/wjg.v10.i10.1499
Revised: September 28, 2003
Accepted: October 7, 2003
Published online: May 15, 2004
AIM: The aim of this study was to describe an auxiliary combined liver-small bowel transplantation model with the preservation of duodenum, head of pancreas and hepatic biliary system in pigs. The technique, feasibility, security and immunosuppression were commented.
METHODS: Forty outbred long-white pigs were randomized into two groups, and the auxiliary composite liver/small bowel allotransplantations were undertaken in 10 long-white pigs in each group with the recipient liver preserved. Group A was not treated with immunosuppressive drugs while group B was treated with cyclosporine A and methylprednisolone after operation. The hemodynamic changes and amylase of body fluid (including blood, urine and abdominal drain) were analyzed.
RESULTS: The average survival time of the animals was 10 ± 1.929 d (6 to 25 d) in group A while more than 30 d in group B. The pigs could tolerate the hemodynamic fluctuation during operation and the hemodynamic parameters recovered to normal 2 h after blood reperfusion. The transient high amylase level was decreased to normal one week after operation and autopsy showed no pancreatitis.
CONCLUSION: Auxiliary en-bloc liver-small bowel transplantation with partial pancreas preservation is a feasible and safe model with simplified surgical techniques for composite liver/small bowel transplantation. This model may be used as a preclinical training model for clinical transplantation method, clinical liver-small bowel transplan-tation related complication research, basic research including immunosuppressive treatment, organ preservation, acute rejection, chronic rejection, immuno-tolerance and xenotransplantation.
-
Citation: Yin ZY, Ni XD, Jiang F, Li N, Li YS, Wang XM, Li JS. Auxiliary
en-bloc liver-small bowel transplantation with partial pancreas preservation in pigs. World J Gastroenterol 2004; 10(10): 1499-1503 - URL: https://www.wjgnet.com/1007-9327/full/v10/i10/1499.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i10.1499
Combined liver and small bowel transplantation (LSBT) is a life-saving procedure in patients especially in children with intestinal failure and parenteral nutrition-related end-stage liver diseases[1]. Recently, due to success in clinical technology and improvement of the operative modality, the American Health Care Financing Administration (HCFA) has granted combined liver-intestinal transplantation as the standard therapeutic modality for patients with irreversible intestinal failure[2]. Patients undergoing composite liver-small bowel transplantation are mostly children. As conventional LSBT requires a loop of defunctionalized (Roux) allograft small bowel for biliary drainage[3], its posttransplant biliary complications including anastomotic leaks and obstruction in 12% of cases, were significantly related with morbidity and mortality[4]. In addition, the conventional composite transplantation needs more vascular anastomoses which would lead not only to more complications including vascular thrombosis but also more difficulties of the surgical techniques. In the present study, we described an auxiliary transplantation technique for LSBT by preserving duodenum, partial head of pancreas and hepatobiliary system in pigs. Experience with this technique and the immunosuppressive treatment for LSBT in pig has not been reported in the literature.
Forty outbred long-white pigs weighing 20-40 kg were divided into 2 groups with random sex, and 10 LSBTs were undertaken in each group. The weight of the donor was generally lower than that of the corresponding recipient. Group A was not treated with immunosuppressive drugs while group B was treated by immunosuppressive therapy consisting of cyclosporine A and methylprednisolone after operation.
The animals were not allowed to eat for 24 h and to drink for 4 h before operation respectively. Gut decontamination was attempted in all donors with an oral antibiotic preparation 3 d before surgery.
After anesthesia with 25 mg/kg of intravenous pentobarbital sodium, the animals were intubated and mechanically ventilated with a mixture of oxygen, nitrous oxide and isoflurane. In addition, standard intravenous antibiotic prophylaxis was instituted with cefotaxime at the time of surgery.
The procurement varied in details but followed the standard techniques for human multiorgan retrieval and our previous report[5-8].
Briefly, the proximal 3 to 4 m of jejunum (the total length of porcine small bowel is about 15 m) together with the liver was procured as the graft. After the redundant small bowel and the dissociative colon were removed from the operative field, the duodenum, proximal jejunum and aorta could be well exposed. An extensive Kocher maneuver allowed visualization of the inferior vena cava and its branch. The subhepatic vena cava was dissected and encircled. The left and right renal veins were ligated respectively. The superior mesenteric and celiac arteries were identified after extending dissection of the aorta. The right and left renal arteries were then isolated and ligated. The subrenal aorta was isolated and encircled distally for the eventual insertion of an infusion cannula. The abdominal aorta was also encircled above the celiac axis for later crossclamping when cold fluid was infused through the distal aortic cannula[9]. The splenic vein was then freed and prepared for portal perfusion cannulation after division of the splenic artery. The splenic vein should be well protected when dividing the splenic artery[10].
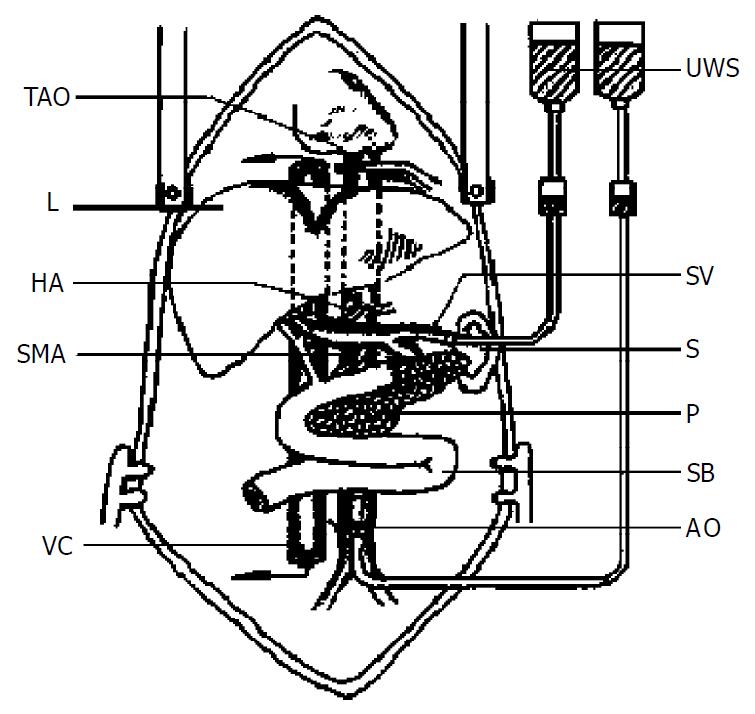
After completion of the preliminary dissection and collection of the donor’s blood, the liver and small bowel connected by the portal vein and aortic segment were lavaged in situ with UW solution (Figure 1). The donor was fully heparinized and the previously encircled proximal aorta was crossclamped, and the distal donor aorta was cannulated with infusion of cold UW solution. For simultaneous portal venous infusion, a venous cannula was placed into the splenic vein and infused with the UW solution. The intrapericardial inferior vena cava and subhepatic vena cava were transected to decompress the infused solution as in other reports[11,12]. The amount of infusion was variable (50-100 mL/kg). After infusion, the graft containing liver, hepatic hilus, pancreatic-duodeno complex, spleen together with splenic vein, and small bowel was achieved with preservation of a segment of aorta containing the superior mesenteric artery and celiac trunk in continuity. The intestine was entrapped by stapling its two ends and carried with the specimen throughout the preservation. Thus the graft was en bloc removed and stored in UW solution at 0 to 4 °C.
Back table procedure was performed in the cold UW solution. It included suturing the orifice of suprahepatic vena cava and the proximal end of the aorta. The spleen was removed. The body and tail of the pancreas along the portal vein were isolated and transected, leaving partial pancreatic head attached to the allograft duodenum. This preserved the superior and inferior pancreatic duodenal arcades. The stump of the pancreas was stapled and then oversewn with a running suture using 4-0 polypropylene.
After anesthesia, a monitor was placed on the recipient. Two venous catheters (with one Swan-Ganz catheter) were inserted for transfusion and hemodynamic parameters including central veinous pressure (CVP), right ventricle pressure (VP), cardiac output (CO), pulmonary artery wedge pressure (PAWP) at the time of preoperation, the time just before blood reperfusion, 5 min, 30 min, 1 h and 2 h after blood reperfusion. The arterial blood pressure was monitored through a femoral artery catheter. The amylase levels of the blood, urine and abdominal drainage postoperative d 1, 3, 5,7 were collected (determined by Somogyi method).
When the recipient’s abdomen was opened, the dissociative colon and its mesentery were dissected and removed to ensure enough celiac space for the composite graft. Then, the end of the ascending colon and the end of the ileum were anastomosed directly to maintain the gastroenterologic continuity of the recipient.
The subrenal aorta was exposed and encircled 2 cm under the renal artery. Small arterial and lymphatic vessels along the aorta were ligated to avoid later bleeding or lymphorrhea. Subhepatic vena cava was also dissected and encircled. The subrenal vena cava was used for the donor out-flow anastomosis.
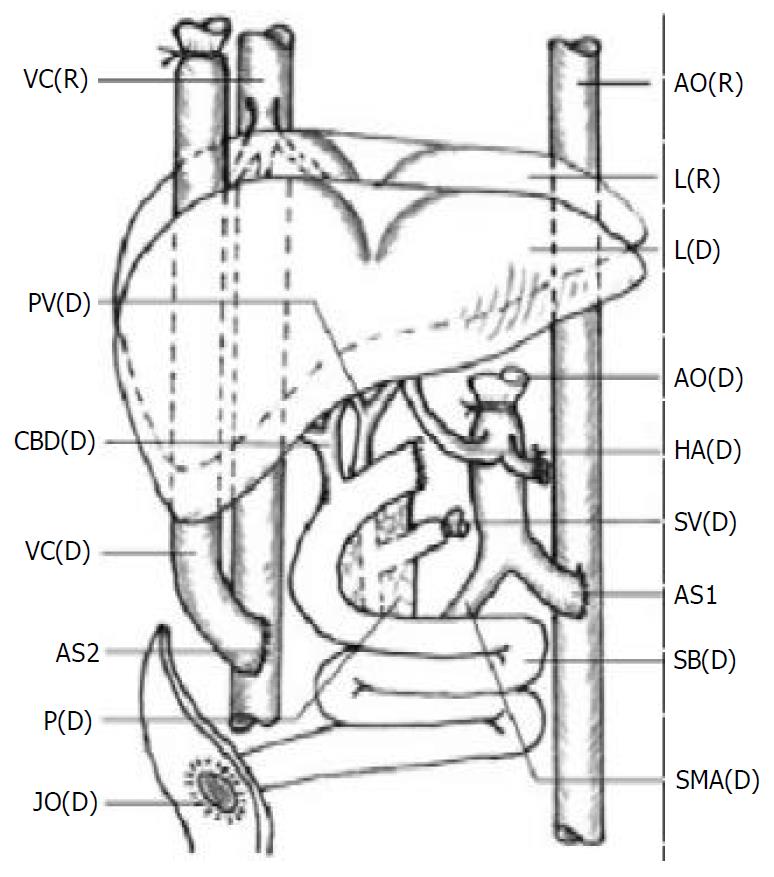
The transplantation methods varied in details but followed the principles as described previously[6,8] (Figure 2).
The arterial inflow was created via an end-to-side anasto-mosis of the graft aorta to the subrenal native aorta with a running polypropylene suture. Donor and recipient vena cava were anastomosed end to side. The venous outflow was the anastomosis of the end of the graft subhepatic vena cava to the side of the native subhepatic vena cava.
Before reperfusion, unclamping and perfusion of the allograft liver were achieved after a lavage of 300 to 500 mL donor blood or Ringer’s solution through the splenic vein. The end of the spleen vein was ligated after the lavage.
The proximal duodenum was closed. A jejunostomy was made at the end of donor distal intestine for early decompression and surveillance endoscopy as described in some clinical reports[13,14].
After operation, the animals were returned to the monitor room, where hemodynamic monitoring and mechanical ventilation were performed as needed 24 h after operation. Due to the high rate of inflammatory complications, broad-spectrum antibacterial prophylaxis was administered for 5 d. Lactated Ringer’s solution and parenteral nutrition were given daily for 2 d after operation.
Immunosurppressive drugs were used in the pigs of group B. Cyclosporine A was started at 15 mg/kg·d by venous injection in the first week, then reduced to 5 mg/kg·d from the second week as maintenance treatment. Methylprednisolone, used for induction and maintenance, was started with 500 mg at the first 24 h postoperation, reduced by 50% to about 2 mg/kg·d for the first week, then reduced by 50% on d 8 and again on day 15.
The survived animals were sacrificed for autopsy 30 d after operation.
After reperfusion, the liver was soft and pink with bile production, evidenced by jejunostomy drainage. If the liver was harder than normal, the outflow of the liver might be obstructed and the vena cava anastomosis needed to be checked. The small bowel should be perfused well, with good mesenteric arterial inflow and venous outflow. The peristalsis and intraluminal mucus production were evident within 15 min after reperfusion.
During the first three days, the intestinal graft stoma appeared healthy, and the mucosa was pink, moist, and well vascularized. No intestinal edema was found in most cases with output of bile-stained stool.
Histopathologic studies of the grafts showed no significant preservation injury. None of the biopsies obtained in the first postoperative week had histological evidence of submucosal bacterial invasion. The frequent cause of death of the pigs in group A was postoperative rejection evidenced by graft pathological examinations when the animal was dead.
Animals died 24 h after reperfusion were ruled out from the statistic series. One pig died because of operative techniques in group A, and all other animals in groups A and B lived for more than 24 h. The average survival time was 10.33 ± 1.929 d (6 to 25 d) in group A while more than 30 d in group B. The difference was significant by Student’s t test (P < 0.01).
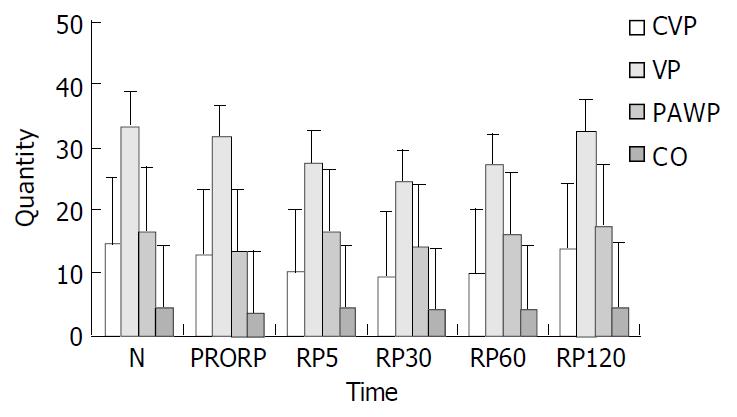
The hemodynamic changes are shown in Table 1. The results suggested that all hemodynamic parameters including CVP, CO, VP and PAWP were decreased during operation, but recovered very quickly after blood reperfusion, and returned to normal 2 h after blood reperfusion as shown in Figure 3.
| POD | 0 | 1 | 3 | 5 | 7 |
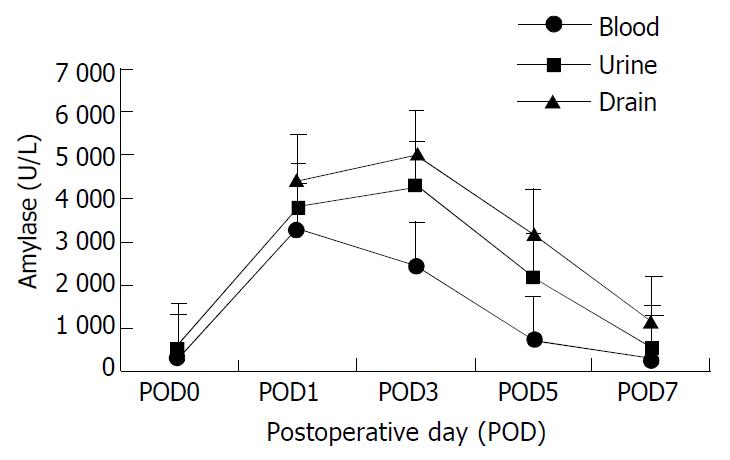
| Blood | 329.60 ± 28.31 | 3314.70 ± 415.29 | 2448.60 ± 413.53 | 718.70 ± 103.61 | 327.70 ± 27.58 |
| Urine | 514.80 ± 55.67 | 3404.80 ± 335.96 | 4307.40 ± 429.16 | 2187.00 ± 148.43 | 491.40 ± 48.80 |
| Drain | _ | 4444.80 ± 545.67 | 5023.80 ± 472.64 | 3217.20 ± 213.91 | 1177.10 ± 98.12 |
Neither the duodenal allografts had signs of ischemia or stump leakage, nor any biliary complication. Blood, urine and abdominal drains were monitored serially for amylase level. Chemical pancreatitis was observed during the early postoperative period with amylase-rich fluid drainage.
The amylases in the body fluid are shown in Table 2. Amylases in blood, urine and abdominal drainage increased promptly after operation and reached their peak in the first three postoperative days. The levels then decreased with time and returned to preoperative level after one week as shown in Figure 4. Postoperative biopsy showed no sign of pancreatitis and also autopsy did not show any evidence of severe pancreatitis or calcification. Autopsy of the pigs in group B showed no evidence of graft rejection.
| Time | N | preRP | RP5 | RP30 | RP60 | RP120 |
| CVP (cmH2O) | 14.80 ± 0.42 | 13.00 ± 0.49 | 10.20 ± 1.07 | 9.50 ± 0.27 | 9.90 ± 0.37 | 14.00 ± 0.36 |
| VP (cmH2O) | 33.70 ± 2.09 | 31.70 ± 2.64 | 27.70 ± 1.93 | 24.60 ± 1.65 | 27.30 ± 1.71 | 32.60 ± 1.92 |
| PAWP (cmH2O) | 16.70 ± 0.59 | 13.40 ± 0.40 | 16.30 ± 0.52 | 14.10 ± 0.41 | 15.90 ± 0.46 | 17.20 ± 0.44 |
| CO (L/min) | 4.48 ± 0.15 | 3.36 ± 0.22 | 4.27 ± 0.15 | 3.98 ± 0.15 | 4.23 ± 0.15 | 4.59 ± 0.16 |
Total parenteral nutrition (TPN) for short bowel syndrome would induce end-stage liver diseases. In considering intestional transplantation for treatment, 60% to 70% them required simultaneous liver allografts[4,7]. Although many clinical liver/small bowel transplantations (LSBT) were reported from different medical centers[15-17], it remains an experimental procedure[18]. Although large animals that share physiological and anatomical similarities with humans, comparing to the widely used rat LSBT model[19,20], large animal models such as LSBT in pigs and its related research were rarely reported.
As compared to the five anastomoses in conventional composite liver-small bowel transplantation including suprahepatic vena cava anastomosis, subhepatic vena cava anastomosis, hepatic artery anastomosis, portal vein anastomosis and bile duct anastomosis, only two anastomoses including aorta-aorta anastomosis and subhepatic vena cava anastomosis were needed in the auxiliary composite liver-small bowel transplantation model. This model could not only decrease possible complications related to the anastomoses such as bleeding, vascular obstruction, postoperative thromboses and bile leakage, but also simplify the surgical techniques. In this model, the vena cava anastomosis was modified by replacing the major hepatic vein (or suprahepatic vena cava) anastomosis in classical Piggy-back transplantation by the subhepatic vena cava anastomosis. This modification has at least two advantages in porcine LSBT. One is that subhepatic vena cava anastomosis can be easily performed, the other is that subhepatic vena cava anastomosis can adjust a flexible anastomotic interval to ensure the aorta-aorta anastomosis easier.
In clinical practice, Abu-Elmagd suggested that LSBT with preservation of pancreatic head and duodenum had some advantages including avoidance of biliary complications and simplification of the operative procedure[20]. These possible advantages were also shown in animal LSBT.
The LSBT transplant procedure is a much more arduous surgical endeavor. The technique of retaining duodenum and the head of pancreas would simplify the back table preparation and avoid risks associated with dissection of the donor hepatic hilus.
Retrieval for composite grafts using the standard technique involved an obligatory reconstruction of the biliary system with a defunctionalized loop of proximal allograft jejunum[3]. In this porcine LSBT model, no biliary reconstruction was required, so that the source of complications such as bile leakage, biliary tract stricture or even death of recipient could be avoided. Liver transplantation related biliary complication rate was about 12%, which would result in about 19% of mortality[21,22]. LSBT with preservation of partial pancreas and duodenum would remarkably decrease such complications.
The potential benefits of any intestinal segment which was kept in direct continuity with alimentary tract freeing the pig for TPN would be enhanced without donor or recipient bowel for Roux-en-Y biliary reconstruction.The advantage of the composite technique was to maintain the hepatic hilus. The use of liver artery and superior mesenteric artery with a large arterial conduit would minimize the risk of hepatic artery thrombosis compared to isolated graft[13,23].
The porcine retrohepatic vena cava is passing through the liver parenchyma with numerous small hepatic veins in this segment besides the major hepatic veins. It is dangerous to remove the liver parenchyma by dissecting the retrohepatic vena cava with high risk of fetal blood loss. This is the reason why the classic piggyback liver transplantation is not suitable in pig. Typical orthotopic liver transplantation in combined LSBT needs not only blood shunt during operation but also a segment of additional artery to prolong the aorta to ensure the arterial anastomosis in pigs. Auxiliary LSBT could avoid the disadvantages of these transplantations.
In conventional composite liver-small bowel transplantation, blood shunt was always needed in the process of vascular exclusion. In pigs, this was much more important because the blood passing through portal vein was accounted for more than 60% of the total porcine blood. The vascular exclusion would lead to suddent cardic failure and death of the pigs. Auxiliary composite liver-small bowel transplantation model with partial vascular exclusion during the vascular anastomosis did not lead to irreversible homodynamic changes that would occur without blood shunt. This method would also avoid other possible hemodynamic damages such as kidney injuries, postoperative thrombsis and ischemia of the lower limb.
Inclusion of duodenum and pancreatic head to maintain continuity of the biliary system was associated with early postoperative allograft pancreatitis, but no significant mortality was reported[24]. This complication was also found in our study. It could be detected by measuring amylase and lipase in peritoneal fluid from abdominal drains, blood and urine[25]. In our study, chemical pancreatitis as shown in the experiment was always transient. The elevated amylase level would return to normal within one week and autopsy showed no signs of severe panceratitis. At present, the following measures could be considered to protect the allografted pancreas from postoperative pancreatitis, namely to limit the amount and pressure of the cold solution used in perfusion[3], to procure the pancreas en-bloc with the graft and avoid over-dissection of the tissue and vessels around duodenum and pancreas[26], to ligate the pancreatic duct and suture the pancreatic interface efficiently and definitely.
The optimal immunosuppressive treatment was not determined in big organ transplantations. Triple drugs (cyclosporine or FK506, azathioprine and prednisolone) and quadruple drugs (ATG, cyclosporine or FK506, azathioprine and prednisolone) treatment in pig transplantation were reported in the literature[27-29]. But the optimal dose of immunosuppressive drugs was ascertained in previous reports. The suggested dose of cyclosporine was from 3 mg/kg·d to 25 mg/kg·d by IV or SB injection[27,28,30,31]. Some reports indicated that pigs required a higher dose of cyclosporine than humans to prevent allograft rejection in thymus transplantation[32]. Other authors suggested that immunosuppr-essive protocols that were successful in preventing allograft rejection in humans had no long-term effect on allografted pigs and the dose of most immunosuppressive agents administered to control rejection in pigs should be higher than that used in humans[33]. This might be due to differences of absorption, binding, metabolism and excretion of the drugs in pigs. So we used the maintenance dose of CsA 5 mg/kg·d after a loading dose of 15 mg/kg·d, and the composite transplanted liver small bowel graft with preservation of pancreas head could be protected by CsA and methylprednisolone against acute rejection.
Some reports suggested that LSBT with preservation of duodenum and pancreatic head would neither increase the possibility of rejection nor require more immunosuppressive treatment than standard LSBT without preservation of pancreas and duodenum[13,23]. The presence of allograft pancreas in multivisceral allografts was not an important risk factor for mortality, and the incidence of rejection of pancreatic was only 12% in some report[13]. So the technique with preservation of the pancreas should be considered safe in composite LSBT.
In summary, auxiliary en-bloc liver-small bowel transplanta-tion with partial pancreas preservation is a feasible and safe model with simplified surgical techniques for composite liver/small bowel transplantation in pigs. This model might be used as a preclinical training model for clinical transplantation method, clinical liver-small bowel transplantation related complication research, basic research including immunosuppressive treatment, organ preservation, acute rejection, chronic rejection, immuno-tolerance and xenotransplantation research.
Edited by Xu JY and Wang XL Proofread by Xu FM
| 1. | Lacaille F, Canioni D, Fournet JC, Revillon Y, Cezard JP, Goulet O. Centrilobular necrosis in children after combined liver and small bowel transplantation. Transplantation. 2002;73:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Abu-Elmagd K, Bond G. The current status and future outlook of intestinal transplantation. Minerva Chir. 2002;57:543-560. [PubMed] |
| 3. | Furukawa H, K Aubu-Elmagd K, Reyes JL. Technical aspects of intestinal transplantation, in: Braverman MH, Tawas RL, (eds): Surgical Technology International II, San Francisco CA. TF Laszlo. 1994;165-170. |
| 4. | Reyes J, Bueno J, Kocoshis S, Green M, Abu-Elmagd K, Furukawa H, Barksdale EM, Strom S, Fung JJ, Todo S. Current status of intestinal transplantation in children. J Pediatr Surg. 1998;33:243-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 154] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Starzl TE, Todo S, Tzakis A, Alessiani M, Casavilla A, Abu-Elmagd K, Fung JJ. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172:335-344. [PubMed] |
| 6. | Casavilla A, Selby R, Abu-Elmagd K, Tzakis A, Todo S, Reyes J, Fung J, Starzl TE. Logistics and technique for combined hepatic-intestinal retrieval. Ann Surg. 1992;216:605-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Williams JW, Sankary HN, Foster PF. Technique for splanchnic transplantation. J Pediatr Surg. 1991;26:79-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Yin ZY, Ni XD, Jiang F, Li N, Li YS, Li JS. Modified technique for combined liver-small bowel transplantation in pigs. World J Gastroenterol. 2003;9:1625-1628. [PubMed] |
| 9. | Abu-Elmagd K, Fung J, Bueno J, Martin D, Madariaga JR, Mazariegos G, Bond G, Molmenti E, Corry RJ, Starzl TE. Logistics and technique for procurement of intestinal, pancreatic, and hepatic grafts from the same donor. Ann Surg. 2000;232:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 158] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | de Ville de Goyet J, Mitchell A, Mayer AD, Beath SV, McKiernan PJ, Kelly DA, Mirza D, Buckles JA. En block combined reduced-liver and small bowel transplants: from large donors to small children. Transplantation. 2000;69:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Starzl TE, Hakala TR, Shaw BW, Hardesty RL, Rosenthal TJ, Griffith BP, Iwatsuki S, Bahnson HT. A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223-230. [PubMed] |
| 12. | Starzl TE, Miller C, Broznick B, Makowka L. An improved technique for multiple organ harvesting. Surg Gynecol Obstet. 1987;165:343-348. [PubMed] |
| 13. | Reyes J, Fishbein T, Bueno J, Mazariegos G, Abu-Elmagd K. Reduced-size orthotopic composite liver-intestinal allograft. Transplantation. 1998;66:489-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Todo S, Tzakis AG, Abu-Elmagd K, Reyes J, Fung JJ, Casavilla A, Nakamura K, Yagihashi A, Jain A, Murase N. Cadaveric small bowel and small bowel-liver transplantation in humans. Transplantation. 1992;53:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 128] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Grant D. Intestinal transplantation: 1997 report of the international registry. Intestinal Transplant Registry. Transplantation. 1999;67:1061-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 241] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Todo S, Reyes J, Furukawa H, Abu-Elmagd K, Lee RG, Tzakis A, Rao AS, Starzl TE. Outcome analysis of 71 clinical intestinal transplantations. Ann Surg. 1995;222:270-280; discussion 280-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 234] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Goulet O, Jan D, Sarnacki S, Brousse N, Colomb V, Salomon R, Cuenod B, Piloquet H, Ricour C, Revillon Y. Isolated and combined liver-small bowel transplantation in Paris: 1987-1995. Transplant Proc. 1996;28:2750. [PubMed] |
| 18. | Muiesan P, Dhawan A, Novelli M, Mieli-Vergani G, Rela M, Heaton ND. Isolated liver transplant and sequential small bowel transplantation for intestinal failure and related liver disease in children. Transplantation. 2000;69:2323-2326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Zhong R, He G, Sakai Y, Zhang Z, Garcia B, Li XC, Jevnikar A, Grant D. The effect of donor-recipient strain combination on rejection and graft-versus-host disease after small bowel/liver transplantation in the rat. Transplantation. 1993;56:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Li XC, Zhong R, He G, Sakai Y, Garcia B, Jevnikar A, Grant D. Host immune suppression after small bowel/liver transplantation in rats. Transpl Int. 1994;7:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Lopez RR, Benner KG, Ivancev K, Keeffe EB, Deveney CW, Pinson CW. Management of biliary complications after liver transplantation. Am J Surg. 1992;163:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Greif F, Bronsther OL, Van Thiel DH, Casavilla A, Iwatsuki S, Tzakis A, Todo S, Fung JJ, Starzl TE. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg. 1994;219:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 357] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 23. | Bueno J, Abu-Elmagd K, Mazariegos G, Madariaga J, Fung J, Reyes J. Composite liver--small bowel allografts with preservation of donor duodenum and hepatic biliary system in children. J Pediatr Surg. 2000;35:291-295; discussion 291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Casavilla A, Selby R, Abu-Elmagd K, Tzakis A, Todo S, Starzl TE. Donor selection and surgical technique for en bloc liver-small bowel graft procurement. Transplant Proc. 1993;25:2622-2623. [PubMed] |
| 25. | Abu-Elmagd K, Reyes J, Todo S, Rao A, Lee R, Irish W, Furukawa H, Bueno J, McMichael J, Fawzy AT. Clinical intestinal transplantation: new perspectives and immunologic considerations. J Am Coll Surg. 1998;186:512-525; discussion 525-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 192] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Kato T, Romero R, Verzaro R, Misiakos E, Khan FA, Pinna AD, Nery JR, Ruiz P, Tzakis AG. Inclusion of entire pancreas in the composite liver and intestinal graft in pediatric intestinal transplantation. Pediatr Transplant. 1999;3:210-214. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Gruessner RW, Fasola C, Fryer J, Nakhleh RE, Kim S, Gruessner AC, Beebe D, Moon C, Troppmann C, Najarian JS. Quadruple immunosuppression in a pig model of small bowel transplantation. J Surg Res. 1996;61:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | McCurry KR, Parker W, Cotterell AH, Weidner BC, Lin SS, Daniels LJ, Holzknecht ZE, Byrne GW, Diamond LE, Logan JS. Humoral responses to pig-to-baboon cardiac transplantation: implications for the pathogenesis and treatment of acute vascular rejection and for accommodation. Hum Immunol. 1997;58:91-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Wennberg L, Groth CG, Tibell A, Zhu S, Liu J, Rafael E, Soderlund J, Bigerfeld P, Morris RE, Karlsson-Parra A. Triple drug treatment with cyclosproine, leflunomide and mycophenolate mofetil prevents rejection of pig islets trans-planted into rats and primates. Transplant Proc. 1997;29:2498. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Alvira LG, Herrera N, Salas C, Pereira F, Herrera J, Suárez-Massa MD, Castillo-Olivares JL. Influence of cyclosporine on graft regeneration and function after liver transplantation: trial in pigs. Transplant Proc. 2002;34:315-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Biffi R, Privitera G, Matinato C, Pozzi S, Marzona L, De Rai P, Andreoni B, Tiberio G, Frezza E, Van Thiel DH. Parenteral antibiotics and selective intestinal decontamination do not prevent enteric bacterial overgrowth or translocation observed in a swine model of small bowel transplantation. J Surg Res. 1995;58:391-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Tuch BE, Wright DC, Martin TE, Keogh GW, Deol HS, Simpson AM, Roach W, Pinto AN. Differentiation of fetal pig endocrine cells after allografting into the thymus gland. Transplantation. 1999;67:1184-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Wright DC, Deol HS, Tuch BE. A comparison of the sensitivity of pig and human peripheral blood mononuclear cells to the antiproliferative effects of traditional and newer immunosuppressive agents. Transpl Immunol. 1999;7:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |