INTRODUCTION
Even 20 years ago, the accepted concept was that one was born with all the pancreatic β cells one ever had[1]. However, now the concept that the pancreatic ductal stem cells exist and can differentiate into pancreatic endocrine cells are generally accepted[2-7]. When the inability of the β cells to match the increased demand for insulin can be seen, diabetes occurs. One of the main obstacles to successful islet transplantation for diabetes is the limitation of available insulin-producing tissue[8]. The lack of pancreatic insulin-producing tissue has given a high priority to efforts to stimulate the growth of pancreatic insulin-producing cells[9-10].
It has been reported that the embryonic stem (ES) cells induce to generate cells expressing insulin and other pancreatic endocrine hormones[11-15]. The cells self-assembled to form three-dimensional clusters similar in topology to normal pancreatic islets where pancreatic cell types were in close association with neurons. Glucose triggered insulin release from these cell clusters by mechanisms similar to those employed in vivo. When injected into diabetic mice, the insulin-producing cells underwent rapid vascularization and maintained a clustered, islet-like organization. Their use might lead to many clinical benefits but it was difficult that the differentiated cells derived from the ES cells were used to assemble functional organs[10,16-21]. It was also shown that the duct tissue from adult human pancreas could be expanded in culture and then was directed to differentiate into glucose responsive islet-like tissue in vitro, but the quantities were limited, the amount would be expected to have little clinical impact[22].
Herein we show it is easier to cultivate human fetal pancreatic ductal stem cells than adult human pancreatic ductal stem cells in vitro and the ductal stem cells can be induced by glucose and differentiate to insulin-producing cells .
MATERIALS AND METHODS
Specimens
The specimens (n = 5) were collected from the Southwest Hospital of the Third Military Medical University. The samples were taken from the spontaneous abortion fetus of the embryonic age 8 stage (about 16-20 wk) of fetus according to the methods of Jirasek[23].
Cell culture
Human fetal pancreas were digested in 1 g/L type IV collagease for 40 min at 37 °C, naturally deposited for 10 min, the supernatant was removed. The cells were then digested in 2.5 g/L trypsin for 30 min at 37 °C, followed by centrifugation for 10 min at 1000 r/min. The deposited cells were put into glucose-free DMEM culture medium containing 2 × non-essential amino acid (NEAA), 1 × B27, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cell suspensions were put into non-treated T-75 flasks having non-sticky glass coverslips inside and incubated at 37 °C in a humidified atmosphere containing 50 mL/L CO2 in air. After 24 h the nonadherent tissue (both viable and dead) was removed, followed by the media change with additional keratinocyte growth factor (KGF, 10 ng/mL, F. Hoffmann-La Roche Ltd, Basel, Switzerland), insulin-transferrin-selenium (ITS, 1 g/L, Sigma-Aldrich China Inc. Shanghai, China), and 2 g/mL BSA, 10 mmol/L nicotinamide (F. Hoffmann-La Roche Ltd, Basel, Switzerland). The adherent or residual cells were continuously cultured and expanded for 5-7 wk by changing the medium every 3 d. The cells were used for identification by immunostaining and further differentiation.
Cell induction and differentiation
For inducing the cells differentiation, the media with or without glucose were subdivided into 4 groups: glucose-free (control), 5 mmol/L, 17.8 mmol/L and 25 mmol/L glucose. Other elements of the media were the same as mentioned above.
Tissue fixation and immunocytochemical staining
Monolayer cells were fixed for 30 min in 40 g/L polyformaldehyde (PFA, in 0.1 mol/L phosphate buffer, pH7.2), and then rinsed in 0.01 mol/L phosphate buffer saline (PBS, pH7.2). The specimens were incubated with 30 mL/L H2O2 in pure methanol for 30 min at room temperature, followed by retrieval 3 times (1 min each time) on a microwave (750 W) in 0.1 mol/L pH6.0 citrate buffer, and incubation with 10 g/L BSA plus 4 g/L Triton X-100 at 37 °C for 30 min. Then immunocytochemical staining was carried out by using primary antibodies of different species: monoclonal mouse anti-human cytokeratin 19 IgG1 antibody (1:100, Santa Cruz Biotechnology, Inc. California, United States), polyclonal rabbit anti-human insulin antibody (H-86, 1:200, Santa Cruz Biotechnology, Inc. California, United States), polyclonal goat anti-human glucagons IgG antibody (C-18, 1:100, Santa Cruz Biotechnology, Inc. California, United States). Horseradish peroxidase-labeled secondary antibody (Sigma-Aldrich China Inc. Shanghai, China) was detected by reaction with 30 mL/L H2O2 and 1 g/L 3, 3’-diaminobenzidine (DAB, Sigma) in solution at room temperature for 5 min, then the specimens were washed and dehydrated in a graded series by ethanol. Finally, the coverslips were mounted cell-side down on glass slides with a drop of DPX and then examined using an Olympus light microscope. Photographs were taken on Lekai-135 film. Positive cells were randomly counted on the montage of photographs generated.
RESULTS
Isolation, cultures and identification of human fetus pancreatic ductal stem cells
To promote the attachment of duct cells rather than islet cells, nonsticky culture flasks were used; these flasks had been used to maintain islets in suspension. The clumps of nonislet tissue could adhere to this nonsticky surface starting about 24 h after culture (Figure 1). Although there was considerable loss of floating tissue of pancreatic acinar tissue in culture, the quantity of cell clumps adhered increased with time. The nonadherent clumps were removed by changing the culture media. In the beginning, the adherent cells were seldom and fusiform. After the nonadherent clumps were removed, the media was changed by serum-free media with additional keratinocyte growth factor to stimulate the growth of only pancreatic ductal stem cells but not the growth of fibroblasts. The cells grew very slowly in the first 2 wk (Figure 2) and 95.2% (975/1024) of the cells were positive for CK-19 immunoreactivity (Figure 3). With additional time of 3 to 4 wk, the cells grew from the adherent clumps and formed monolayer with clear epithelial morphology. The cells were large, cobblestone patterns (Figure 4 and Figure 5). There was a significant increase of the cultured tissue during the 5-6 wk of culture.
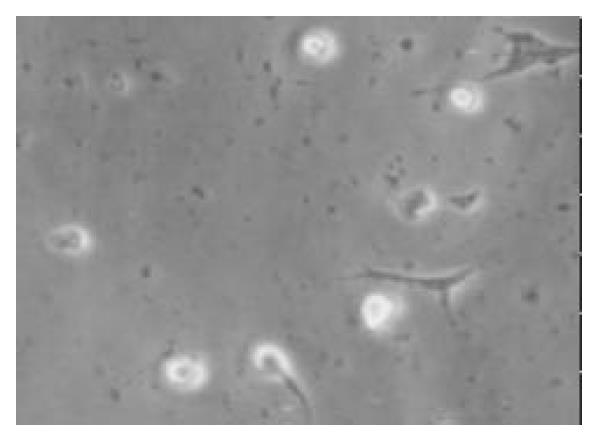
Figure 1 After being cultured for 24 h, a few cells were adherented on the bottom of non-sticky flasks, some of the cells processed protrusions (× 100).
Figure 2 After being cultured for 2 wk in the media added the keratinocyte growth factor, the number of cells increased, the cells became fusiform with a few processes (× 100).
Figure 3 Most cells of culture were positive for CK-19 immu-noreactivity after being cultured for 2 wk (× 100).
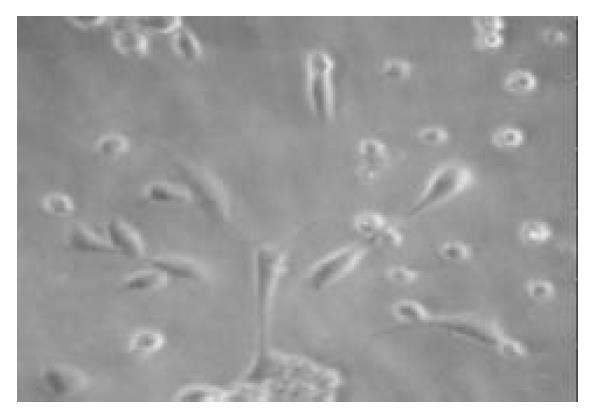
Figure 4 The cells were cobblestone pattern after being cul-tured for 4 wk (× 150).
Figure 5 The cells were cobblestone pattern and formed mono-layer after being cultured for 6 wk (× 100).
Differentiation of pancreatic ductal stem cells into insulin-producing cells
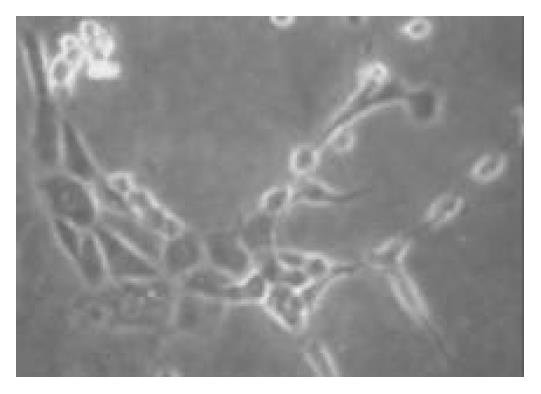
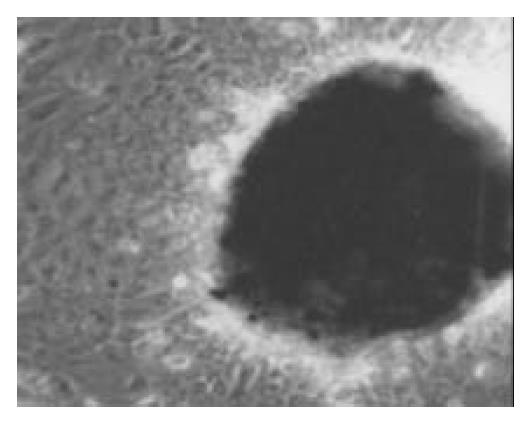
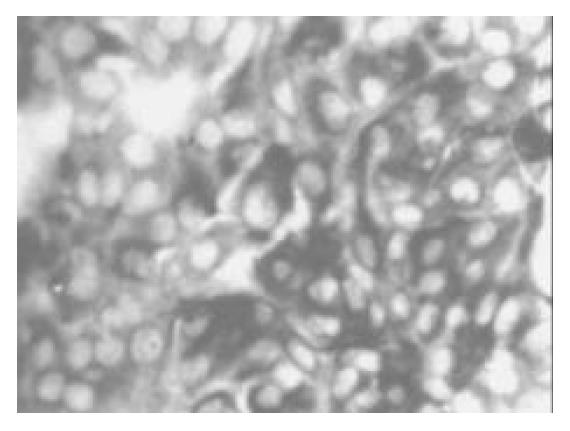
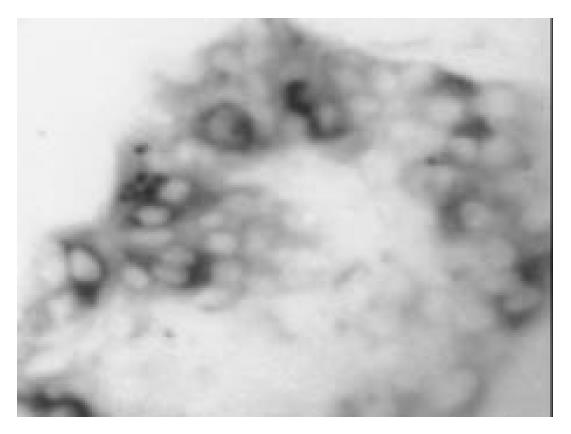
Once the clumps were attached and formed monolayers, the media were changed by adding glucose in different concentration of 0, 5, 17.8 and 25 mmol/L, respectively. Over next 1-2 wk the plaques of epithelial cells became crowded, and formed a few pancreatic islet-like structures (Figure 6). Some of the cells in pancreatic islet-like structures were positive for insulin immunoreactivity (Figure 7), and a few cells were positive for glucagon immunoreactivity (Figure 8).
Figure 6 The cells were induced to form pancreatic islet-like struc-tures after being cultured in the media containing 17.
8 mmol/L glucose for 1 wk (× 100).
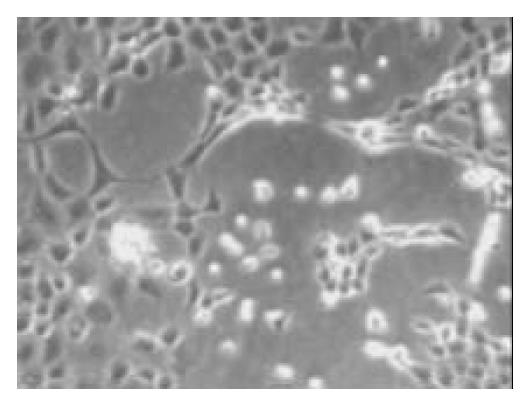
Figure 7 After being cultured in the medium containing 17.
8 mmol/L glucose for 1 wk, many cells in islet-like struc-tures were positve for insulin immunoreactivity (× 150).
Figure 8 After being cultured in the medium containing 17 mmol/L glucose for 1 wk, a few cells in islet-like structures were posi-tive for glucagon immunoreactivity (× 150).
The stainings in immunopositive cells were located in the cytoplasm, but the immunonegative cells also revealed light stainings in the perinuclear region similar to those in control group. The intensity of the immunostaining varied from one cell to another. The shape of the positive cells also varied; most of the cells were polygonal in shape, but spherical or fusiform cells were also observed. The nucleus was unstained, and some cells seemed to have 2 nuclei. Analysis of the cells from randomly generated micrographs of 25 cultured coverslips revealed different percentages of the cells immunoreactivity for CK-19, insulin and glucagons after the cells were induced by different concentration of glucose for 1-2 wk.
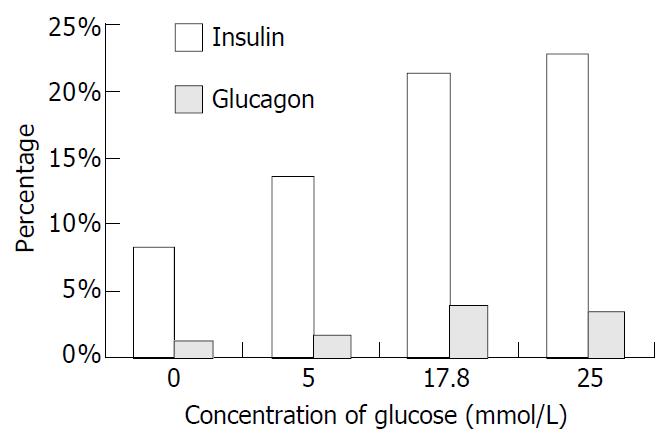
After induction for 2 wk with different concentrations of glucose, the numbers of positive cells for insulin and glucagon were different. The cells were CK-19 immunonegative, while the insulin immunopositive cells were obviously increased. When the concentration of glucose was 17.8 mmol/L, the ratio of insulin positive cells reached a very high level. In the media without glucose, 108 cells of total counting 1325 (8.2%) were positive for insulin immunostaining, and 15 cells of total counting 1187 (1.3%) were positive for glucagons immunostaining. When the concentration of glucose reached 5 mmol/L, the number of insulin-positive cells was 153 of total counting 1142 (13.4%), while the number of glucagons-positive cells was 19 of total counting 1092 (1.7%). In the medium containing 17.8 mmol/L glucose, 230 insulin-positive cells of total counting 1079 (21.3%) and 40 glucagon-positive cells of total counting 1046 (3.8%) were observed. When the concentration of glucose was 25 mmol/L, the insulin-positive cells were 255 of total counting 1125 (22.7%) and the glucagons-positive cells were 39 of total counting 1127 (3.5%) (Figure 9).
Figure 9 Percentage of immunopositive cells induced by glucose.
DISCUSSION
In pancreas, insulin is produced and secreted by specialized structures, islets of Langerhans. Diabetes, which affects thousands of million people in the world, results from abnormal function of pancreatic islets. The main obstacle to successful islet transplantation for diabetes is the limitation of available insulin-producing tissue[24]. Herein, we introduced to generate cells expressing insulin from human fetal pancreas duct tissue. This approach may provide a potential new source of pancreatic islet cells for transplantation.
Most studies have shown there is limited in vitro growth of adult islet cells of any species[25]. From the studies on rat pancreatic regeneration, it inferred that the capacity of adult pancreatic duct cells were strongly to both expand and differentiate[26]. Bonner-Weir et al[27] showed the expansion of adult human pancreatic ductal tissue in vitro and its subsequent differentiation to islet cells after being overlaid with Matrigel, and over 3-4 wk culture there was a significant increase in insulin as well as formation of islet-like structures called cultivated human islet buds. These data further provided proofs for the hypothesis that pancreatic ductal cells have the potential to lose their specific ductal epithelial cell phenotype with rapid proliferation in vitro, reverting to multipotent stem cells that then can differentiate into islet cells with the appropriate external stimuli[28]. But the report showed the amount limitation and associated with loss of insulin production[27]. In order to avoid these problems, we made use of human fetal pancreas from the spontaneous abortion human fetus, combined 2-enzyme digestion with mechanical dissection of the pancreas. The duct tissue could be dissected effectively and the single cells were rich in stem cells. The cell proliferation could be extended for at least 6 wk. The glucose can stimulate the pancreatic ductal stem cells to differentiate into insulin-producing cells.
Now, human islet isolations can yield maximum 400000-600000 islets per donor, which means that more than one donor may be requested for a successful transplant. In our study reported here that the pancreatic ductal stem cells, after in vitro expansion and glucose-induced differentiation, became organized into islet-like structures consisting of insulin- and glucagons-producing cells. Their use may lead to many clinical benefits. Because the pancreatic islets rarely adhered directly to the nonsticky flasks, and the culture medium was changed at time, the culture conditions did not favor the islets inclusion. These data provide evidence of the potential to expand and differentiate human pancreatic ductal stem cells to islet cells in vitro, but further optimization of conditions is needed to generate yields of islet tissue that will make an impact on islet transplantation. We have been able to expand human pancreatic ductal stem cells and then to direct its differentiation into insulin- and glucagons-producing cells, respectively. The ability to cultivate human islets from digested pancreatic ductal stem cells opens a new approach for β cell replacement therapy.
The culture condition’s optimization could include further expansion of the ductal tissue or higher efficiency in differentiating cells. Of the cells adhered in the beginning, the most cells were ductal stem cells, some were fibroblasts. But the culture medium without serum and with keratinocyte growth factor favored the growth of ductal stem cells. The data from rodents suggested that in vitro culture of exocrine tissue (the ducts and acini) would result in ductal epithelial cells. Moreover, mouse pancreatic exocrine (acinar and ductular) tissue gave rise to epithelial cultures that were indistinguishable from cultures of isolated duct, raising the possibility that acinar cells could dedifferentiate to form duct cells[29,30]. It is entirely possible that the cells from the smaller ducts/acini have a little capacity to differentiate into endocrine cells[27]. Other studies suggested that between 50%-95% of the rodent exocrine cells died in the initial culture condition with mainly the ductal stem cells left to replenish the cultures[31].
In our study, the adherent cells during the early culture period seemed to be pancreatic ductal stem cells. The culture conditions were serum-free, glucose-free but including keratinocyte growth factor, and these media stimulated the proliferation of ductal stem cells but not fibroblasts. The CK-19 positive cells that formed in fusiform and cobblestone pattern were characteristic of pancreatic ductal stem cells. In contrast, the insulin-producing cells were CK-19 immunonegative and insulin immunopositive. The glucose could induce the ductal stem cells differentiation into insulin-producing cells, and the ratio of positive cells is dependent on the concentration of glucose.
Our study differs from previous work in several ways. In most other study, the human islets were isolated and expanded on an extracellular matrix substrate, in which islets were easily mixed with the ductal stem cells[27,32,33]. With expansion as monolayers and culture in three-dimensional collagen gels, human islets gradually lost endocrine phenotype[27]. In the study of Bonner-Weir, mainly ductal tissue remaining after islet isolation was expanded and then coated with extracellular matrix to induce differentiation into islet cells[27]. The potential of extracellular matrix to induce differentiation has been shown for other epithelial cell types. In the present study, we selected different concentration of glucose as the inducing element, which effectively induced the fetal ductal stem cells differentiation.
The expansion of duct tissue is effective and extensive, probably because of the removal of normal intercellular restraints found. Because the default pathway of differentiation of embryonic pancreas is thought to be that of islet formation, such restraint might be protective and necessary to prevent excessive islet formation that could produce too much insulin and even hypoglycemia[29,30]. In the present experiment, we are able to generate new islet cells from duct stem cells, but the quantities are limited. Despite the limitations at this early stage, these findings raise the tantalizing possibility that this approach, once optimized, may generate meaningful amounts of new human insulin-producing cells from duct stem cells. This possibility has important implications for making β cell replacement therapy available to a larger number of patients with type 1 and 2 diabetes mellitus.