Published online May 15, 2004. doi: 10.3748/wjg.v10.i10.1440
Revised: April 5, 2004
Accepted: April 9, 2004
Published online: May 15, 2004
AIM: We have previously demonstrated that cholangiocytes, the epithelial cells lining intrahepatic bile ducts, encode two functional bile acid transporters via alternative splicing of a single gene to facilitate bile acid vectorial transport. Cholangiocytes possess ASBT, an apical sodium-dependent bile acid transporter to take up bile acids, and t-ASBT, a basolateral alternatively spliced and truncated form of ASBT to efflux bile acids. Though hepatocyte and ileal bile acid transporters are in part regulated by the flux of bile acids, the effect of alterations in bile acid flux on the expression of t-ASBT in terminal ileocytes remains unclear. Thus, we tested the hypothesis that expression of ASBT and t-ASBT in cholangiocytes and ileocytes was regulated by bile acid flux.
METHODS: Expression of ASBT and t-ASBT message and protein in cholangiocytes and ileocytes isolated from pair-fed rats given control (C) and 1% taurocholate (TCA) or 5% cholestyramine (CY) enriched diets, were assessed by both quantitative RNase protection assays and quantitative immunoblotting. The data obtained from each of the control groups were pooled to reflect the changes observed following TCA and CY treatments with respect to the control diets. Cholangiocyte taurocholate uptake was determined using a novel microperfusion technique on intrahepatic bile duct units (IBDUs) derived from C, TCA and CY fed rats.
RESULTS: In cholangiocytes, both ASBT and t-ASBT message RNA and protein were significantly decreased in response to TCA feeding compared to C diet. In contrast, message and protein of both bile acid transporters significantly increased following CY feeding compared to C diet. In the ileum, TCA feeding significantly up-regulated both ASBT and t-ASBT message and protein compared to C diet, while CY feeding significantly down-regulated message and protein of both bile acid transporters compared to C diet. As anticipated from alterations in cholangiocyte ASBT expression, the uptake of taurocholate in microperfused IBDUs derived from rats on TCA diet decreased 2.7-fold, whereas it increased 1.7-fold in those on CY diet compared to C diet fed groups.
CONCLUSION: These data demonstrate that expression of ASBT and t-ASBT in cholangiocytes is regulated by a negative feedback loop while the expression of these transporters in terminal ileum is modified via positive feedback. Thus, while transcriptional regulatory mechanisms in response to alterations in bile acid pool size are operative in both cholangiocytes and ileocytes, each cell type responds differently to bile acid supplementation and depletion.
- Citation: Kip NS, Lazaridis KN, Masyuk AI, Splinter PL, Huebert RC, LaRusso NF. Differential expression of cholangiocyte and ileal bile acid transporters following bile acid supplementation and depletion. World J Gastroenterol 2004; 10(10): 1440-1446
- URL: https://www.wjgnet.com/1007-9327/full/v10/i10/1440.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i10.1440
Bile acids are synthesized in hepatocytes from cholesterol, conjugated to either taurine or glycine, secreted into bile and reach the small intestine via the bile ducts to facilitate lipid absorption[1]. Subsequently, bile acids are reclaimed at the terminal ileum, returned to the liver via the portal circulation, and resecreted into bile accomplishing their enterohepatic circulation[1]. At the cellular level, this recycling of bile acids, important for conservation of the bile acid pool, is achieved by the coordinated activities of a series of apical and basolateral membrane bile acid transporters expressed on the epithelial cells of the terminal ileum and liver[2].
The active recovery of conjugated bile acids in the terminal ileum is mediated by a Na+ driven bile acid transporter, ASBT, located at the luminal domain of the ileocyte[2]. Following uptake into the ileocytes, bile acids bind to the intestinal bile acid binding protein (I-BABP) in the cytoplasm to be directed across the cell to the basolateral membrane where they enter into the portal venous circulation by a Na+-independent mechanism[2]. Subsequently, the majority of conjugated bile acids are efficiently reabsorbed from portal vein blood into hepatocytes, mainly via the Na+-taurocholate cotransporter (ntcp)[3] and then are secreted into the biliary system primarily via ATP-dependent export pumps located on the apical (i.e., canalicular) domain of hepatocytes[3].
As bile percolates through the intrahepatic bile ducts, it undergoes numerous modifications due to both secretory and absorptive activities of cholangiocytes. We and others, have previously shown that biliary epithelia can take up conjugated bile acids via ASBT[4,5] expressed on the cholangiocyte apical membrane, a protein identical to that cloned from rat ileum and kidney[6,7]. We have also recently identified an alternatively spliced and truncated transcript of ASBT, designated t-ASBT that allows cholangiocytes and possibly other bile-acid transporting epithelia (i.e., ileum and kidney) to extrude bile acids at the basolateral domain[8].
Despite these advances in our understanding of the physiology of bile acid transport, little is known about the regulation of ASBT and t-ASBT in bile acid transporting epithelia, especially in cholangiocytes. While recent studies have assessed the effects of ursodeoxycholic acid supplementation[9], bile acid depletion and repletion in bile duct ligated rats[10,11] and bile acid feeding on different physiologic responses by cholangiocytes, including in some instances ASBT expression[12]. No studies have addressed the regulation of t-ASBT or systematically compared the responses of the liver and ileum to bile acid expansion or depletion. We, therefore, designed the present study to further investigate the role of bile acids in the regulation of ASBT and t-ASBT in both the biliary tree and terminal ileum. For this purpose, we performed studies in non-surgically manipulated rats to determine the message and protein levels of ASBT and t-ASBT in both cholangiocytes and ileocytes following well-accepted experimental perturbations to either expand (i.e., taurocholate feeding) or deplete (i.e., cholestyramine feeding) the bile acid pool. To ensure that our molecular observations were physiologically relevant, we also employed functional studies using microperfusion of rat intrahepatic bile duct units (IBDUs), a technique recently developed in our laboratory.
[α-32P] UTP (specific activity 800 Ci/mmol) of > 95% purity was purchased from Amersham (Arlington Heights, IL), taurocholate (TCA) of 98% purity was obtained from Calbiochem-Novabiochem Corp (La Jolla, CA). MAXIscript SP6/T7 high specific activity transcription kit and RPA III kit were purchased from Ambion Inc (Austin, TX). All sodium dodecyl sulfate-polyacrylamide gel reagents were purchased from Biorad (Hercules, CA). The ASBT antibody used in immunoblotting was a generous gift of Paul Dawson[2]. The t-ASBT antibody was produced as described previously[8]. The secondary antibody (goat anti-rabbit), a horseradish peroxidase (HRP) conjugate, was obtained from Biosource International. Enhanced chemiluminescence (ECL) Western blotting detection reagents were bought from Amersham (Arlington Heights, IL). X-ray films were purchased from Eastman Kodak Co (Rochester, NY). For measurement of biliary phospholipids and cholesterol, commercial kits were purchased from Wako Chemicals (Richmond, VA) and Boehringer Mannheim (Indianapolis, IN). All other commercially available reagents, including cholestyramine, were obtained from Sigma Chemical Co (St. Louis, MO).
All procedures involving rats were in compliance with guidelines of the Mayo Foundation Animal Care Committee, which approved the experimental protocols. Male Fischer 344 rats (225-275 g) purchased from Harlan Sprague Dawley Inc (Indianapolis, IN) were maintained in a temperature-controlled environment (22 °C) with alternating 12-h light-dark cycles. Rats had free access to water but were pair-fed for 10 d, either control diet (C), or diets enriched with 1% taurocholate (TCA) (wt/wt) or 5% cholestyramine (CY) (wt/wt) prepared by Dyets Inc (Bethlehem, PA). The feeding studies were conducted in two paired groups (i.e., control and test) and four sets of rats (TCA vs C and CY vs C). However for data analysis, results from each of the control groups with respect to the message and protein data were pooled. To ensure equal chow consumption between the pair-fed groups, each rat was kept in an individual cage and its food intake and weight were monitored daily. According to the pair-feeding protocol, the amount of chow consumed by each one of the TCA or CY designated rats one day was provided the following day to the corresponding pair-fed C animal as control chow. At the end of each feeding study, rats were anesthetized with sodium pentobarbital (50 mg/kg) intraperitoneally, the common bile duct was cannulated and bile was collected continuously in timed aliquots to determine bile flow rate. Bile samples were extracted with 4 volumes of isopropanol and immediately frozen in liquid nitrogen for later measurements of biliary bile acids, phospholipids and cholesterol. Subsequently, rats were euthanized consistently between 8 and 9 am to control possible circadian variation in ASBT and t-ASBT expression.
Freshly isolated, highly purified cholangiocytes (> 95%) were prepared from the livers of C, TCA, and CY fed rats using collagenase perfusion, enzymatic digestion, mechanical disruption and immunopurification as described[13]. Cholangiocytes derived from animals of the same feeding group were pooled and kept at -70 °C until used for mRNA or protein extraction.
The small intestine was prepped and 15 cm of terminal ileum was excised, flushed with cold 9 g/L NaCl solution and the luminal surface was scraped to harvest the epithelial cells. Ileocytes collected from animals of the same feeding group were pooled, centrifuged at 2000 rpm for 5 min, and the pellet was kept at -70 °C until used for mRNA isolation and protein extraction.
Following extraction of total cellular RNA from freshly isolated cholangiocytes and ileocytes[14], ASBT and t-ASBT mRNA were determined by Q-RPA using the RPA III kit, as previously described[8]. Yeast and kidney RNA were used as a negative and positive control, respectively. Following RPA, the ASBT or t-ASBT mRNA content of the samples was measured both by scanning the X-ray film of the protected bands corresponding to either ASBT or t-ASBT and by comparing their arbitrary densitometric units to those obtained from their respective standard curves as described[8]. The comparability of the total RNA used in this assay was determined both spectrophotometrically and by ultraviolet detection of the intensity of 28S and 14S ribosomal bands on ethidium bromide stained gels.
Following cholangiocyte immunoisolation, the cells were sonicated twice for 7 s, using a Sonifier cell disrupter (Heat Systems-Ultrasonic, Inc. Plainview, NY) in a buffer containing 50 mmol/L Tris-HCl (pH7.4), 50 mmol/L EDTA, 100 μmol/L leupeptin, 1 μmol/L pepstatin and 0.2 mmol/L PMSF. Cell fragments attached to immunomagnetic beads were removed by applying the samples to a magnet. The remaining cell lysate was centrifuged at 4 °C for 10 min at 2500 rpm Subsequently, the cholangiocyte post nuclear supernatant (PNS) was collected, protein concentration was determined and stored at -70 °C until used. To obtain PNS from ileocytes of the terminal ileum, the scraped ileocytes were sonicated in the same buffer and centrifuged as indicated above. In the PNS fraction of ileocytes, protein concentration was calculated and then it was stored at -70 °C until used.
Cholangiocyte and ileal PNS were run on a 120 g/L SDS-polyacrylamide gel. Electrophoresis was done at 100 volts (V) in the presence of a prestained low molecular range protein as a size marker (Biorad) and 40 μg of kidney PNS as a positive control. Proteins were transferred overnight at 4 °C at 25 V onto nitrocellulose membranes (Micron Seperations Inc., Westboro, MA) as described[4]. Immunoblotting of ASBT and t-ASBT was performed using the well-characterized, specific, rabbit anti rat, polyclonal anti-ASBT[6] and anti-t-ASBT[8] antibodies. In brief, membranes were blocked in 50 mL/L milk and incubated at room temperature for 2 h with ASBT antibody (1:4000) or t-ASBT antibody (1:1000). Subsequently, membranes were washed 3 times and exposed to horseradish peroxidase conjugated secondary (goat anti-rabbit) antibody (1:7000) for 1 h at room temperature. Following three additional washings of the membrane, the immunoreactive ASBT (48 kDa) and t-ASBT (19 kDa) bands were detected by enhanced chemiluminescence detection system (ECL).
Immunoblotting for β-actin (41 kDa), a housekeeping protein, was employed to demonstrate equal protein loading. Quantitative comparisons among experimental groups were made by determining the immunoreactive areas by densitometry using an imaging densitometer (Model GS-700) and the Molecular Analyst software program (Bio-Rad Laboratories, Hercules, CA) to calculate the ratios of ASBT or t-ASBT to that of β-actin. The immunoblotting data were also evaluated using another technique to exclude possible risk of β-actin being altered by any of the experimental perturbations. For this purpose, arbitrary densitometric units of each immunoreactive band were divided by the μg of protein loaded and this value was further divided by the exposure time of the autoradiograph. The specificity of ASBT and t-ASBT antibodies and the bands observed in the immunoblots were evaluated by stripping the membranes and reprobing them with ASBT and t-ASBT antibodies (3 mg/mL) previously blocked with their respective peptides (2 mg/mL) for 1 h at 37°C[4,8].
Isolation and microperfusion of IBDUs were performed, as previously described[15]. Microperfusion of an IBDU was carried out using Ringer-HCO3 (KRB solution) containing 40 mmol/L taurocholate at a perfusion rate of 436 nl/min and bile acid (i.e., taurocholate) uptake by individual microperfused IBDU was calculated as published previously[15].
Concentrations of proteins were measured by the fluorescamine method using bovine serum albumin (BSA) as standard[16]. Data were confirmed using the colorimetric Bradford method where vendor’s (Sigma) instructions were applied. No differences were observed in protein recovery in TCA or CY groups when compared to controls for a specific cell type.
Biliary phospholipids and cholesterol were analyzed spectrophotometrically using commercial kits from Wako Chemicals and Boehringer Mannheim, respectively. Biliary bile acid concentration was determined spectrophotometrically according to the vendor’s instructions (Sigma). Bile acid concentration of samples was derived from a calibration curve prepared using bile acid of known concentrations: 5, 10, 50, 100 and 200 μmol/L.
All data were expressed as mean ± SE. Statistical differences were analyzed by Student’s t-test, and results were considered to be statistically different at P < 0.05.
The effects of TCA and CY feeding on rat body mass, bile flow and biliary lipid composition compared to C diet are provided in Table 1. Though an approximate 6% weight loss was observed following TCA and CY feedings, the differences in total body masses in experimental groups vs C diet were not significant. Bile flow was significantly higher (2.2-fold) in the TCA group, but similar in the CY group compared to the C group. As expected, the biliary bile acids in the TCA fed rats were 3.2-fold higher, but 2-fold lower in the CY fed rats compared to the C fed rats. Biliary phospholipids of the TCA fed rats were significantly greater. In the CY group, biliary phospholipids were significantly lower than in the C group. Finally, biliary cholesterol levels of both the TCA and CY groups were similar compared to the C rats. These data indicated that the diets employed significantly altered bile flow and the biliary lipid parameters as anticipated.
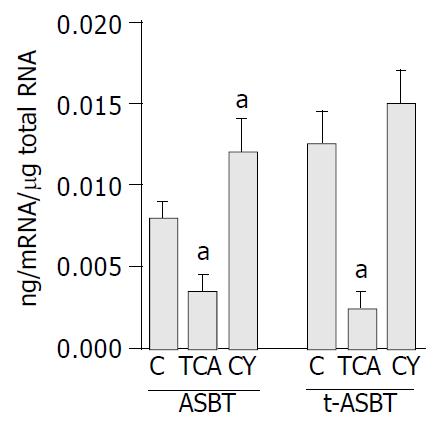
Using Q-RPA, ASBT mRNA levels were significantly lower (2.3-fold) in cholangiocytes of the TCA fed rats than in cholangiocytes of the C fed rats (0.0035 ± 0.002 ng mRNA/μg total RNA and 0.0081 ± 0.002 ng mRNA/μg total RNA, respectively, P = 0.009) (Figure 1). Parallel to the down-regulation of ASBT in the TCA fed rats, t-ASBT mRNA of the same group was also 4.3-fold lower compared to the C fed rats (0.003 ± 0.001 ng mRNA/μg total RNA and 0.013 ± 0.003 ng mRNA/μg total RNA, respectively, P = 0.01) (Figure 1). ASBT mRNA levels were 1.5-fold higher in cholangiocytes of the CY fed rats than in those of the C fed rats (0.012 ± 0.002 ng mRNA/μg total RNA and 0.0081 ± 0.002 ng, RNA/μg total RNA, respectively, P = 0.04) (Figure 1). Corresponding to the upregulation of ASBT in the CY fed rats, t-ASBT mRNA of the same group was also 1.3-fold greater compared to the C fed rats. However, this was not statistically significant (0.015 ± 0.004 ng mRNA/μg total RNA and 0.013 ± 0.003 ng mRNA/μg total RNA, respectively, P = 0.4) (Figure 1). The insignificant increase in the t-ASBT message and the significant increase in the protein levels of t-ASBT (Figure 2) following CY treatment might indicate post-transcriptional modifications.
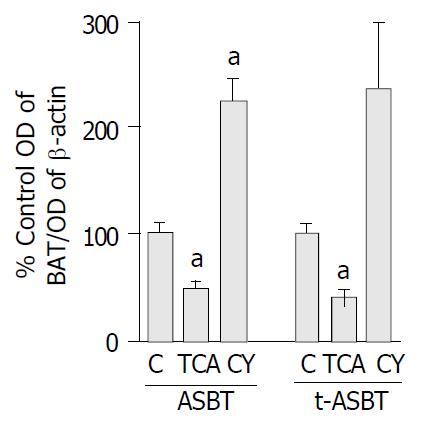
ASBT protein in the TCA group decreased 2.2-fold compared to the C fed rats and that was statistically significant (P = 0.006) (Figure 2). Protein levels of t-ASBT were also repressed 2.4-fold following TCA feeding compared to C fed rats that was also statistically significant (P = 0.004) (Figure 2). The protein quantitative data were also analyzed using an additional approach to verify the above findings. In this case, to exclude the possibility of β-actin being affected by the bile acid feeding, quantitative analysis was made by determining the arbitrary densitometric units of each immunoreactive band and further dividing it by the amount of protein loaded and exposure time of the autoradiograph. Using this alternative approach, cholangiocyte ASBT protein from the TCA group was shown to be downregulated 2.2-fold compared to C fed rats. This finding reassured us about the accuracy of the data we obtained by the first quantitative method and provided evidence that actin was not affected by TCA feeding. In addition, following CY feeding, the ASBT protein was 2.3-fold higher and statistically significant compared to C fed rats (P = 0.003) (Figure 2). Moreover, after CY feeding, the t-ASBT protein was also increased 2.4-fold compared to C fed rats (P = 0.04) (Figure 2).
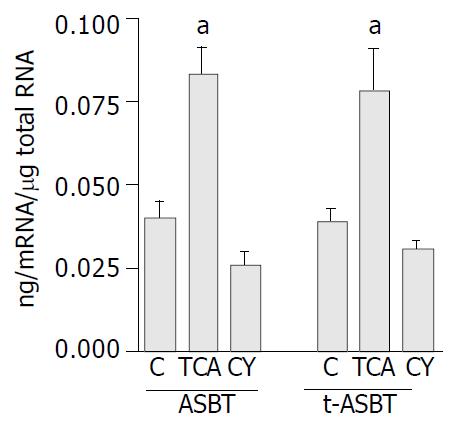
Using Q-RPA, ASBT mRNA levels in ileum of the TCA fed rats were 2.1-fold higher than those in ileum of the C fed rats (0.073 ± 0.01 ng mRNA/μg total RNA and 0.036 ± 0.01 ng mRNA/μg total RNA, respectively, P = 0.01) (Figure 3). Parallel to upregulation of ASBT message, t-ASBT mRNA of the TCA group was also 1.9 fold higher compared to the C fed rats (0.076 ± 0.015 ng mRNA/μg total RNA and 0.040 ± 0.004 ng mRNA/μg total RNA, respectively, P = 0.01) (Figure 3). In addition, the ileal ASBT mRNA levels of the CY fed rats were 1.4-fold less and statistically significant than those of the C fed rats (0.026 ± 0.003 ng mRNA/μg total RNA and 0.036 ± 0.007 ng mRNA/μg total RNA, respectively, P = 0.07) (Figure 3). Corresponding to these findings, t-ASBT mRNA levels of the CY fed rats were 1.3-fold lower and statistically significant compared to the C fed rats (0.031 ± 0.002 ng mRNA/μg total RNA and 0.040 ± 0.004 ng mRNA/μg total RNA, P = 0.01) (Figure 3).
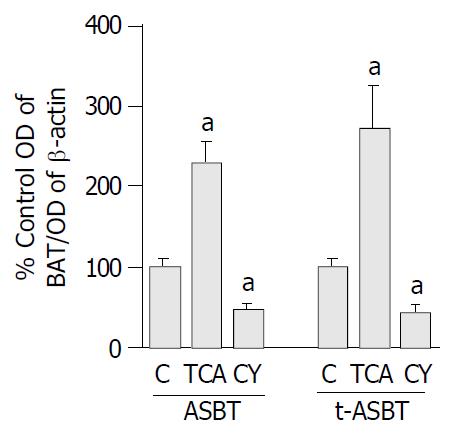
Parallel to increased ASBT transcript observed following bile acid feeding, ASBT protein expression was also 2.3-fold higher and statistically significant in the TCA fed rats compared to the C fed rats (P = 0.001) (Figure 4). Moreover, the t-ASBT protein expression was also supportive of the mRNA data, t-ASBT protein was 2.7-fold higher and statistically significant in the TCA fed rats compared to the C fed rats (P = 0.02) (Figure 4). Furthermore, following CY feeding, and parallel to decreased ASBT transcript, ileal ASBT protein was also 2.1-fold significantly down-regulated (P = 0.008) compared to the C fed rats (Figure 4). Finally, the ileal t-ASBT protein was also in support of the message results for this transporter. Indeed, t-ASBT protein was 2.3-fold significantly decreased in the CY fed rats compared to the C fed rats (P = 0.01) (Figure 4).
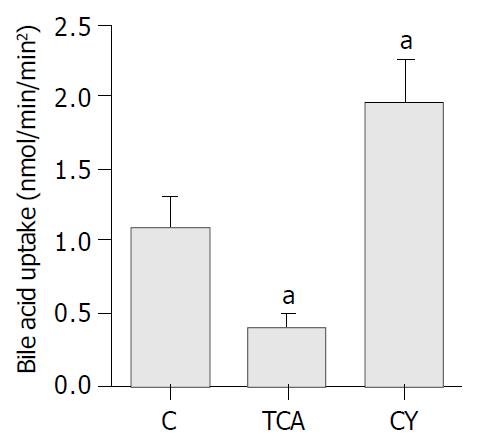
To further verify and extend our observations at the functional level, taurocholate uptake was measured in IBDUs isolated from rats fed either TCA or CY diets and compared to those obtained from C fed animals using a novel microperfusion technique. Specifically, taurocholate uptake by IBDUs derived from the C diet group was 1.1 ± 0.2 nmol/min/mm2. Taurocholate uptake was significantly lower in the TCA group (0.4 ± 0.1 nmol/min/mm2, P = 0.002, n = 4 IBDU, 2 feeding studies) and significantly higher in CY group (1.95 ± 0.3 nmol/min/mm2, P = 0.007) (Figure 5).
The major findings described here are TCA feeding downregulates but CY feeding upregulates both ASBT and t-ASBT in cholangiocytes and TCA feeding upregulates whereas CY feeding downregulates both ASBT and t-ASBT in terminal ileum. Our findings suggested that both ASBT and t-ASBT in a specific cell type underwent parallel modes of regulation. On the other hand, reciprocal changes were observed between cholangiocytes and ileocytes in terms of ASBT and t-ASBT expression, suggesting organ-specific differences in regulation of these bile acid transporters.
Regulation of transporter expression to accommodate altered substrate loads is a well-recognized physiologic phenomenon. For example, adaptive up- or down- regulation of hepatic and ileal bile acid transporters occurred in response to changes in bile acid pool size[3]. Moreover, a limited number of very recent studies addressed the problem of whether the same adaptive events could operate in cholangiocytes with respect to ASBT[9-12]. However, no data exist regarding the regulation of t-ASBT in the liver or terminal ileum after bile acid supplementation or depletion. Regarding ASBT expression in terminal ileum, prior investigations have shown evidence of transcriptional regulation by bile acids[17-19]. Nevertheless, results of these studies appeared to be species-specific given reported differences in ASBT regulation. For example, Lillienau et al[17] reported a negative feedback regulation of the ileal bile acid transporter in the guinea pig based on whole organ, ileal perfusion studies. Following cloning of ASBT in rats, Stravitz et al[18] described a positive feedback regulatory mechanism of the ileal ASBT. These authors demonstrated induction of both mRNA and protein levels of ASBT after cholic acid feeding including supportive functional studies using ileal brush border membrane vesicles. More recently, Arrese et al[19] reported no change in ASBT expression and function following bile duct ligation and intestinal sequestration of bile acids.
In the present study, we demonstrated that in cholangiocytes compared to C fed rats, TCA feeding downregulated both ASBT transcript and protein abundance as well as t-ASBT transcript and protein expression. On the other hand, CY feeding upregulated both ASBT transcript and protein abundance as well as t-ASBT transcript and protein expression compared to C fed rats. To support these molecular findings, microperfusion studies in rat IBDUs compared to C fed animals revealed 2.7-fold decrease and 1.7-fold increase in taurocholate uptake by IBDUs derived from TCA or CY fed rats, respectively. In terminal ileum, TCA feeding upregulated both ASBT transcript and protein abundance as well as t-ASBT transcript and protein expression. In contrast, CY feeding downregulated both ileal ASBT transcript and protein abundance as well as t-ASBT transcript and protein expression compared to C fed rats.
The pathophysiological significance of ASBT and t-ASBT adaptive changes in biliary epithelia and terminal ileum in response to bile acid pool perturbations described in this paper are intriguing. It appears that bile acid transporting epithelia of different organs (i.e., bile ducts and ileum) respond differently to bile acid supplementation or depletion. ASBT and t-ASBT transcriptional down-regulation in cholangiocytes upon TCA feeding could diminish the cholehepatic shunt of conjugated bile acids. This pathway is believed to play a role in reclamation of bile acids from bile back to liver. Thus, during bile acid feeding, decreased expression of bile acid transporters in cholangiocytes and the ensuing reduction of bile acid shunting may provide protection to cholangiocytes and subsequently hepatocytes from the deleterious effects of excessive intracellular accumulation of bile acids. In fact, this protective mechanism became even more relevant given the fact that bile acid feeding increased the expression of bile acid transporters in terminal ileum as shown in the present and other studies[18] and thus enhancing the uptake capacity of ileum for bile acids for delivery to the liver, perhaps to compensate the decreased cholehepatic shunt, and thus keeping the bile acid synthesis at a balance.
We have also observed that in CY fed rats, cholangiocyte ASBT and t-ASBT were up-regulated. These data should be reviewed in parallel with the reciprocal findings in the terminal ileum where CY feeding caused upregulation of both ASBT and t-ASBT. The potential pathophysiologic relevance of ASBT and t-ASBT induction in cholangiocytes after CY feeding may relate to increasing the biliary bile acid return to the liver via an enhanced cholehapatic shunt. This condition would potentially assist maintaining cholesterol homeostasis, given the concurrent observation of diminished expression of bile acid transporters in distal intestine and thus reducing absorption of bile acids from the terminal ileum. The experimental effect of CY on the expression of cholangiocyte bile acid transporters is analogous to the bile duct ligated (BDL) model, in which the enterohepatic circulation of bile acids is interrupted due to mechanical obstruction of the common bile duct. To this end, our observations in cholangiocytes were comparable to those reported by Lee et al[11]. These authors reported adaptive up-regulation and down-regulation of ASBT in biliary epithelia and kidney, respectively, in BDL rats, postulating that these changes might accommodate alternative pathways for secretion of excessive bile acids in cholestasis[11]. However, others have shown in both BDL[9,10] and bile acid fed rats[12] that bile acid depletion and repletion could decrease and increase the expression of ASBT, respectively, in cholangiocytes. Differences in results may very well reflect differences in study design (e.g., feeding rats in a paired vs non-paired manner, feeding them until the surgical procedure vs fasting them overnight, feeding for 10 d vs 7 d, the age of the animals 225-275 g vs 125-150 g starting masses).
In our ileal studies, a significant increase in ASBT and t-ASBT occurred after TCA feeding, whereas a decrease in message and protein levels of both transporters was seen in bile acid depleted rats. These data suggest that changes in bile acid pool size could result in marked alterations in these bile acid carrier molecules. Our data agree with the work of Stravitz et al[18] who also reported a positive feedback mechanism on the regulation of ASBT in ileum. Arrese et al[19] reported that neither the pharmacological sequestration of bile acids nor common bile duct ligation affected the expression and function of ileal ASBT. Whether the different pharmacological agents used for bile acid depletion by Arrese (i.e., the novel sequestrant GT31-104)[19], and in our work, the well-established cholestyramine, accounted for these differences is unclear[18].
Recently, the promoter region of the ASBT gene has been extensively analyzed[20]. It appears that two AP-1 consensus sites have been identified in the proximal promoter of ASBT. At least four nuclear binding proteins, designated ABP 1-4, interacting with AP-1 sites, have been described. Of interest, formation of the ABP2 nuclear binding protein complex appeared to be correlated with regional and developmental stage-specific expression of ASBT in the rat intestine[20]. In a study clarifying the transcriptional mechanisms involved in cytokine-mediated repression of rat ASBT, the authors observed reductions in ileal ASBT message and protein following proinflammatory cytokine and IL-1 beta treatment of IEC-6 and Caco-2 cells, along with significant increases in c-fos expression[22]. The proinflammatory cytokines and interleukin-1 beta have thus been shown to repress the activity of the ASBT promoter via serine phosphorylation, and nuclear translocation of c-fos. Inflammation, associated with up-regulation, phosphorylation, and nuclear translocation of c-fos, which then represses ASBT promoter activity via binding to the 3’ AP-1 element by a c-fos/c-jun heterodimer, may be operative following bile acid treatment, a stimulus known to increase inflammation in cholangiocytes. The inflammatory influence of bile acids might occur following directly activating eosinophils and inducing their effector functions[23], or triggering interferon-gamma and tumor necrosis factor alpha production, which might in turn stimulate superoxide and interleukin release that resulted in disruption of the tight junctions in cholangiocytes[24]. Bile acids, with the potential of altering the profile of cytokines, chemokines, and proinflammatory stimuli, which in turn were likely to activate fibrogenesis, stimulate apoptotic and proliferative responses, seemed to be important players in the pathophysiology of chronic cholestatic disorders[25]. It is therefore imperative to begin to investigate the role of AP-1, ABP 1-4 and c-fos expression in bile acid transporting epithelia hoping to shed light on the adaptive and reciprocal expression of ASBT and t-ASBT. In a recent study, decreased expression of ileal ASBT protein and mRNA in mice following bile acid treatment was related to the presence of a potential liver receptor homologue-1 (LRH-1) cis acting element in the bile acid responsive region of the mouse but not the rat promoter. The mouse but not the rat promoter was activated by LRH-1 and this correlated with nuclear protein binding to the mouse but not rat LRH-1 element. Thus, species-specific negative feedback regulation of ASBT by bile acids was mediated by FXR via SHP-dependent repression of LRH-1 activation of the ASBT promoter[21]. It is possible, therefore that, the reciprocal regulation pattern we have observed between cholangiocytes and ileocytes may reflect the role of FXR and LRH-1 in cholangiocytes.
In conclusion, we present data demonstrate that a bile acid enriched diet can down-regulate ASBT and t-ASBT in cholangiocytes but upregulate both transporters in terminal ileum. Conversely, bile acid depletion by sequestration can upregulate ASBT and t-ASBT in cholangiocytes, but down-regulate both transporters in terminal ileum. To have a better understanding of the mechanism (s) affecting the expression of bile acid transporters in relevant epithelia, a systematic comparative evaluation of their regulatory elements in different tissues known to be involved in bile acid homeostasis is required. Unraveling the presumed cell-specific regulatory mechanisms that affect bile acid transporter expression and function will help to develop novel rational options to treat cholestasis and chronic cholestatic liver diseases.
Edited by Wang XL Proofread by Xu FM
| 1. | Hofmann AF. Intestinal absorption of bile acids and biliary constituents: the intestinal component of the enterohepatic cir-culation and the integrated system. New York: Raven 1994; 1845-1865. |
| 2. | Dawson PA, Oelkers P. Bile acid transporters. Curr Opin Lipidol. 1995;6:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | St-Pierre MV, Kullak-Ublick GA, Hagenbuch B, Meier PJ. Transport of bile acids in hepatic and non-hepatic tissues. J Exp Biol. 2001;204:1673-1686. [PubMed] |
| 4. | Lazaridis KN, Pham L, Tietz P, Marinelli RA, deGroen PC, Levine S, Dawson PA, LaRusso NF. Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J Clin Invest. 1997;100:2714-2721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 184] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Alpini G, Glaser SS, Rodgers R, Phinizy JL, Robertson WE, Lasater J, Caligiuri A, Tretjak Z, LeSage GD. Functional expression of the apical Na+-dependent bile acid transporter in large but not small rat cholangiocytes. Gastroenterology. 1997;113:1734-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 119] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Shneider BL, Dawson PA, Christie DM, Hardikar W, Wong MH, Suchy FJ. Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. J Clin Invest. 1995;95:745-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 230] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Christie DM, Dawson PA, Thevananther S, Shneider BL. Comparative analysis of the ontogeny of a sodium-dependent bile acid transporter in rat kidney and ileum. Am J Physiol. 1996;271:G377-G385. [PubMed] |
| 8. | Lazaridis KN, Tietz P, Wu T, Kip S, Dawson PA, LaRusso NF. Alternative splicing of the rat sodium/bile acid transporter changes its cellular localization and transport properties. Proc Natl Acad Sci USA. 2000;97:11092-11097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Alpini G, Baiocchi L, Glaser S, Ueno Y, Marzioni M, Francis H, Phinizy JL, Angelico M, Lesage G. Ursodeoxycholate and tauroursodeoxycholate inhibit cholangiocyte growth and secretion of BDL rats through activation of PKC alpha. Hepatology. 2002;35:1041-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Alpini G, Glaser S, Alvaro D, Ueno Y, Marzioni M, Francis H, Baiocchi L, Stati T, Barbaro B, Phinizy JL. Bile acid depletion and repletion regulate cholangiocyte growth and secretion by a phosphatidylinositol 3-kinase-dependent pathway in rats. Gastroenterology. 2002;123:1226-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Lee J, Azzaroli F, Wang L, Soroka CJ, Gigliozzi A, Setchell KD, Kramer W, Boyer JL. Adaptive regulation of bile salt transporters in kidney and liver in obstructive cholestasis in the rat. Gastroenterology. 2001;121:1473-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Alpini G, Ueno Y, Glaser SS, Marzioni M, Phinizy JL, Francis H, Lesage G. Bile acid feeding increased proliferative activity and apical bile acid transporter expression in both small and large rat cholangiocytes. Hepatology. 2001;34:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Alpini G, Phillips JO, Vroman B, LaRusso NF. Recent advances in the isolation of liver cells. Hepatology. 1994;20:494-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 105] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40518] [Cited by in RCA: 39099] [Article Influence: 1028.9] [Reference Citation Analysis (0)] |
| 15. | Masyuk AI, Gong AY, Kip S, Burke MJ, LaRusso NF. Perfused rat intrahepatic bile ducts secrete and absorb water, solute, and ions. Gastroenterology. 2000;119:1672-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Udenfriend S, Stein S, Böhlen P, Dairman W, Leimgruber W, Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972;178:871-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2129] [Cited by in RCA: 1987] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 17. | Lillienau J, Crombie DL, Munoz J, Longmire-Cook SJ, Hagey LR, Hofmann AF. Negative feedback regulation of the ileal bile acid transport system in rodents. Gastroenterology. 1993;104:38-46. [PubMed] |
| 18. | Stravitz RT, Sanyal AJ, Pandak WM, Vlahcevic ZR, Beets JW, Dawson PA. Induction of sodium-dependent bile acid transporter messenger RNA, protein, and activity in rat ileum by cholic acid. Gastroenterology. 1997;113:1599-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Arrese M, Trauner M, Sacchiero RJ, Crossman MW, Shneider BL. Neither intestinal sequestration of bile acids nor common bile duct ligation modulate the expression and function of the rat ileal bile acid transporter. Hepatology. 1998;28:1081-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Chen F, Ma L, Al-Ansari N, Shneider B. The role of AP-1 in the transcriptional regulation of the rat apical sodium-dependent bile acid transporter. J Biol Chem. 2001;276:38703-38714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Chen F, Ma L, Dawson PA, Sinal CJ, Sehayek E, Gonzalez FJ, Breslow J, Ananthanarayanan M, Shneider BL. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J Biol Chem. 2003;278:19909-19916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 189] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Chen F, Ma L, Sartor RB, Li F, Xiong H, Sun AQ, Shneider B. Inflammatory-mediated repression of the rat ileal sodium-dependent bile acid transporter by c-fos nuclear translocation. Gastroenterology. 2002;123:2005-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Yamazaki K, Gleich GJ, Kita H. Bile acids induce eosinophil degranulation by two different mechanisms. Hepatology. 2001;33:582-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Hanada S, Harada M, Koga H, Kawaguchi T, Taniguchi E, Kumashiro R, Ueno T, Ueno Y, Ishii M, Sakisaka S. Tumor necrosis factor-alpha and interferon-gamma directly impair epithelial barrier function in cultured mouse cholangiocytes. Liver Int. 2003;23:3-11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Strazzabosco M. Transport systems in cholangiocytes: their role in bile formation and cholestasis. Yale J Biol Med. 1997;70:427-434. [PubMed] |