Published online May 15, 2004. doi: 10.3748/wjg.v10.i10.1421
Revised: February 18, 2004
Accepted: February 21, 2004
Published online: May 15, 2004
AIM: To transfect murine angiostatin cDNA into human hepatocellular carcinoma cell line SMMC-7721 and to investigate its effects on implanted carcinoma in nude mice.
METHODS: A eukaryotic expression vector of pcDNA3.1-mAST containing murine angiostatin was constructed. Then pcDNA3.1-mAST plasmid was transfected into cell line SMMC-7721 by Lipofectamine. The resistant clone was screened by G418 filtration and identified by RT-PCR and Western blotting. Nude mice were divided into three groups of 10 each. Mice in blank control group were only injected with SMMC-7721 cells. Mice in vector control group were injected with SMMC-7721 cells transfected with pcDNA3.1 (+) vector, whereas mice in angiostatin group were injected with SMMC-7721 cells transfected with pcDNA3.1-mAST plasmid. Volume, mass and microvessel density (MVD) of the tumors in different groups were measured and compared.
RESULTS: Murine angiostatin cDNA was successfully cloned into the eukaryotic expression vector pcDNA3.1 (+). pcDNA3.1-mAST was successfully transfected into SMMC-7721 cell line and showed stable expression in this cell line. No significant difference was observed in the growth speed of SMMC-7721 cells between groups transfected with and without angiostatin cDNA. Tumor volume, mass and MVD in the angiostatin group were significantly lower than those in the blank control group and vector control group (P < 0.01). The inhibitory rate of tumor reached 78.6%. Mass and MVD of the tumors only accounted for 34.6% and 48.9% respectively of those in the blank control group.
CONCLUSION: Angiostatin cDNA could be stably expressed in human hepatocellular carcinoma cell line SMMC-7721 without obvious inhibitory effects on the growth of SMMC-7721 cells. When implanted into nude mice, SMMC-7721 cells transfected with angiostatin cDNA show a decreased tumorigenic capability. It suggests that angiostatin can inhibit tumor growth through its inhibition on angiogenesis in tumors.
- Citation: Tao KS, Dou KF, Wu XA. Expression of angiostatin cDNA in human hepatocellular carcinoma cell line SMMC-7721 and its effect on implanted carcinoma in nude mice. World J Gastroenterol 2004; 10(10): 1421-1424
- URL: https://www.wjgnet.com/1007-9327/full/v10/i10/1421.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i10.1421
The growth and metastasis of a tumor depend on the growth of blood vessels[1-5], so anti-angiogenesis becoming a new way in treatment of tumors[6-9]. Recently, angiostatin has been found to be one of the most effective inhibitory genes of angiogenesis. It can inhibit specifically the proliferation and migration of endothelial cells of blood vessels, and has been regarded as a very useful target gene in anti-angiogenesis-based cancer treatment[10]. Liver cancer is one kind of cancers rich in blood vessels, so therapeutic angiogenesis represents a potential option in the therapy of primary liver cancer. We investigated the construction of an expression vector containing angiostatin cDNA and evaluated its effect on implanted tumor in nude mice.
Eukaryotic expression vector of pcDNA3.1 (+) (Invitrogen, USA) and plasmid pRC-mAST containing full-length of angiostatin gene (a gift from Dr. Zhang DX in Folkman Laboratory, Yale University, USA) were digested by restriction enzymes Xba I and Hind III. The digested vector and angiostatin cDNA fragment were ligated. Recombinant clones were identified by Xba I and Hind III double digestion. Positive clones named pcDNA3.1-mAST were further confirmed by sequencing.
Cultured SMMC-7721 cells were divided into three groups: transfected with recombinant pcDNA3.1-mAST (group A), transfected with pcDNA3.1 (+) vector (group B), not transfected with pcDNA3.1 (+) vector (group C). Transfection was performed according to the instructions of Lipofectamine TM2000 reagent kit (Gibco). Cells were cultured in RPMI 1640 medium containing 200 mL/L fetal calf serum and 350 mg/L G418. Resistant clones could be detected two weeks later. Cell growth curve was also made.
Total RNA was extracted following reversal transcriptase PCR. Primers used in PCR were designed according to the reported angiostatin cDNA sequence[7]. The primer sequences were as follows: 5’ end: 5’-ATGGACCATAAGGAAGTAA-3’; 3’ end 5’-GGTGGGCAATTCCACAAACA-3’. The products of PCR were identified on 10 g/L agarose gel electrophoresis.
Cultured cells in the three groups were treated by adding 500 L solution containing 500 g/L lysine-Sepharose and 50 mmol/L Tris-HCl (pH8.0) into the culture medium. Western blotting was then performed according to the reported methods[11]. The primary antibody was anti-rabbit HA-tagged antibody (a gift from Dr. Zhang DX in Yale University).
Thirty Balb/c nu/nu male mice aged 4-6 wk (body mass 18-20 g) were bred under SPF conditions. They were randomly divided into three groups of 10 each. The mice in blank control group were injected only with SMMC-7721 cells, the mice in vector-treated control group were injected with SMMC-7721 cells transfected with pcDNA3.1 (+) vector, the mice in angiostatin group received an injection of SMMC-7721 cells transfected with recombinant pcDNA3.1-mAST. After cancer cells were cultured into the stage of logarithmic growth phase, they were digested with trypsin to make cancer cell suspension of 5 × 1010/L. Then, 0.2 mL of each suspension was subcutaneously injected into the right back of nude mice.
The survival of nude mice was observed every day. Tumor volume and inhibitory rate were measured on days 7, 14, 21, 28 and 35 after injection.
Tumor volume = π/6 × (long radius × short radius2)[12].
Inhibition rate = (tumor volume of blank control group-tumor volume of angiostatin group)/tumor volume of blank control group × 100%.
Thirty-five days after cancer cell injection, the nude mice were killed and their tumors were removed. The surrounding fatty tissues were dissected and the tumors were weighed. CD34 immunohistochemical staining was carried out according to the previously reported methods[11] to label endothelial cells of blood vessels. Five areas with the highest microvessel density (MVD) in each section were selected under 40 × subjective lens. The number of blood vessels in each area was counted under a magnification of 200 filds (0.708 mm2/field). The data from 5 areas were averaged and the value was regarded as the tumor MVD of each nude mouse. The average MVD from 10 mice in each group was regarded as the MVD of implanted tumor of that group[13].
All data were expressed as mean ± SD and analyzed by Student’s t test. A P value less than 0.05 was considered statistically significant.
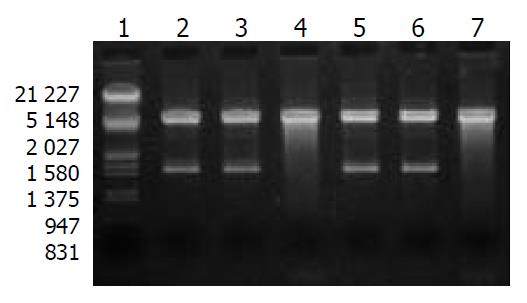
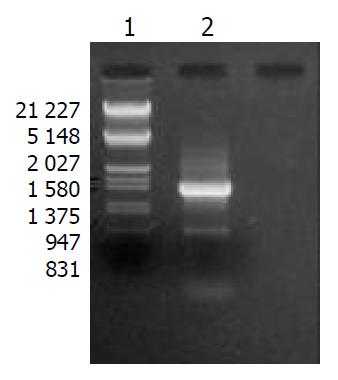
After digestion by Xba I and Hind III, bands at 1.4 kb could be detected for positive clones (Figure 1), which suggested that mAST fragment was inserted into the pcDNA3.1 (+) vector, named recombinant plasmid pcDNA3.1-mAST.
pcDNA3.1-mAST plasmid DNA was prepared and performed for sequencing. The sequence obtained was the same as the reported sequence of angiostatin cDNA, indicating that the murine angiostatin gene was successfully cloned into the eukaryotic expression vector pcDNA3.1 (+).
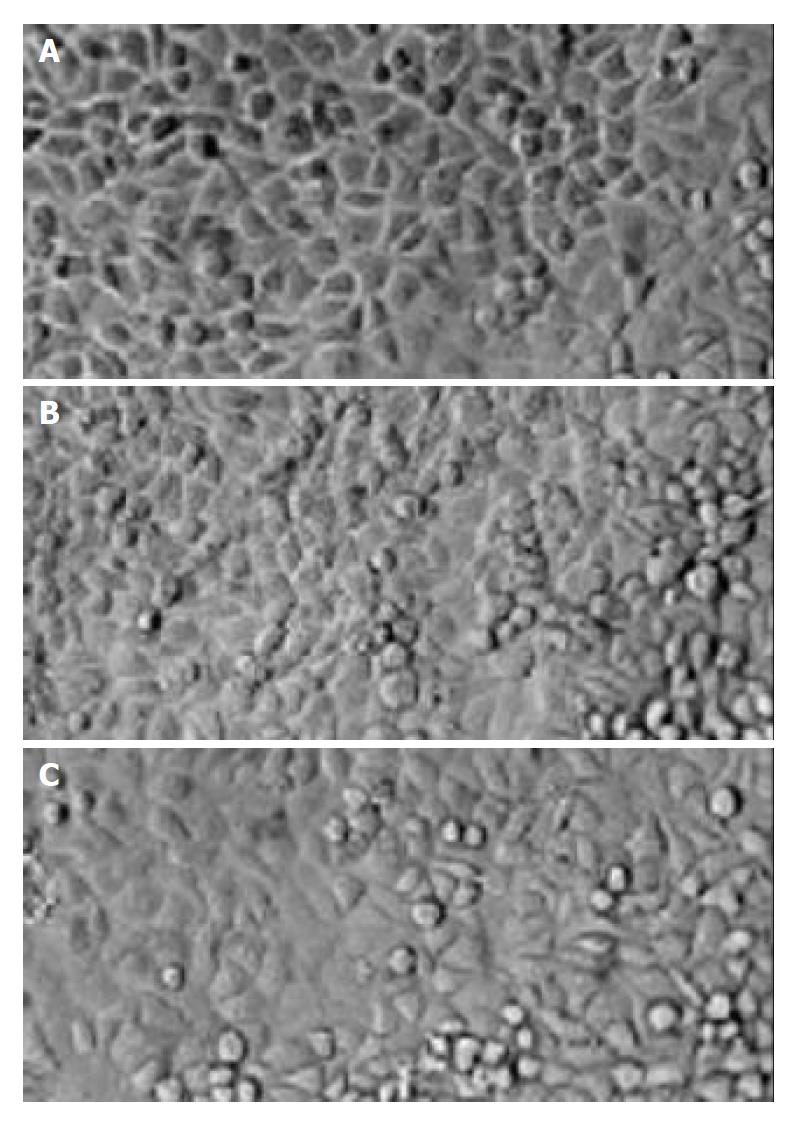
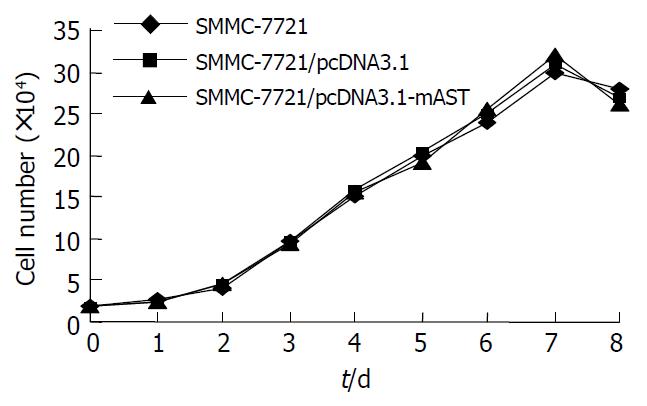
No significant differences between the morphological characteristics of transfected cells and normal SMCC-7721cells (Figure 2) were observed. No differences were detected in their growth rates (Figure 3).
SMMC-7721 cells transfected with pcDNA3.1-mAST were prepared and used as the template. By using angiostatin primers, a band was detected at 1.4 kb with PCR, indicating the presence of angiostatin cDNA in SMMC-7721 liver cancer cells (Figure 4).
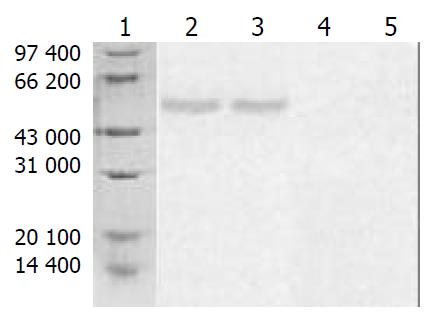
Supernatants of cultured cells in three groups were collected and analyzed by Western blotting. A band in a molecular mass of 58000 was detected with rabbit anti-HA-tagged antibody from the cells transfected with pcDNA3.1-mAST, but no bands were detected from blank control group or vector control group (Figure 5).
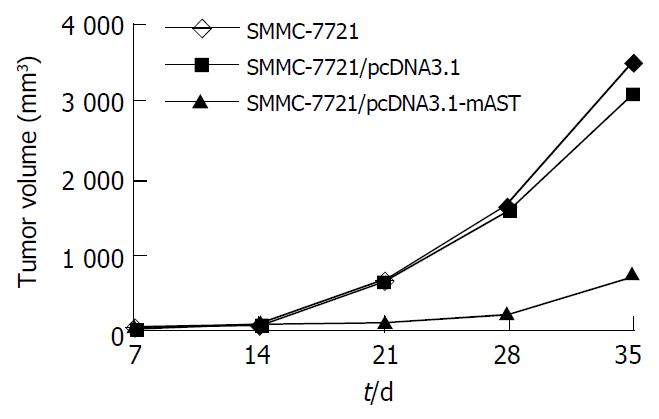
Tumors were observed in the nude mice just 5 d after they were implanted with cells in blank control group or vector control group. There was no significant difference in tumor volume between blank control group and vector control group (t = 1.53, P > 0.05). Mice had a visible tumor 10 d after cell injection in pcDNA3.1-mAST transfection group, and the tumor grew slowly. Tumor volumes among three groups were quite different, and a significant difference was observed when compared angiostatin group with blank control group or vector control group (t = 13.07 and t = 12.91, respectively, P < 0.01, Table 1).
| Group | Time after implantation (d) | ||||
| 7 | 14 | 21 | 28 | 35 | |
| Blank control | 20 ± 5 | 91 ± 25 | 624 ± 139 | 1631 ± 363 | 3538 ± 643 |
| Vector control | 19 ± 6 | 85 ± 24 | 653 ± 149 | 1542 ± 358 | 3128 ± 547 |
| Angiostatin | 0 | 23 ± 6 | 112 ± 20 | 237 ± 46 | 755 ± 198b |
On the 35th day, the tumor growth inhibition rate in angiostatin group reached 78.6% vs the control group. In addition, the speed of tumor growth in angiostatin group was significantly slower than that in blank control or vector control group (Figure 6).
On the other hand, the tumor mass in three groups was measured on day 35. It was found that the tumor mass in angiostatin group (2.1 ± 0.5 g) was significantly smaller than that in blank control (6.0 ± 0.7 g) or vector control (5.9 ± 0.5 g) group (t = 14.98 and 16.14, respectively, P < 0.01), whereas there was no significant difference in tumor mass between the blank control and vector control groups (t = 0.59, P > 0.05).
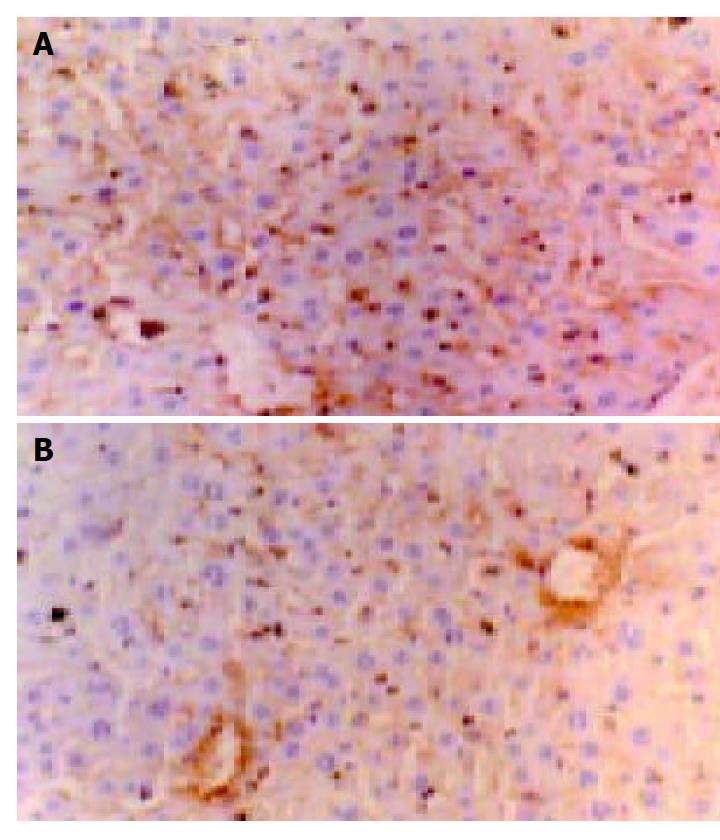
Blood vessels were visualized by CD34 immunohistochemistry and endothelial cells were stained in brown color (Figure 7). There were no significant differences of MVD between the vector control group (49 ± 7, mm2) and the blank control group (52 ± 6, mm2) (t = 0.92, P > 0.05). But the tumor MVD in the angiostatin group (26 ± 4, mm2) was significantly lower than that in other two control groups (t = 9.33, 10.94, respectively, P < 0.01), and was about 48.9% of that in the blank control group.
The growth and metastasis of a tumor depend upon the growth of blood vessels. Therefore, inhibition of blood vessel growth is a potential therapy for primary tumors, and it has become an important way to treat tumors by increasing the expression of inhibitory factors of angiogenesis in the tumor area. Angiostatin is one of the endogenous angiogenesis inhibitory factors[10] and has been proved in vitro to inhibit the proliferation of endothelial cells. In vivo studies also confirmed that angiostatin could inhibit the angiogenesis in solid tumors that could result in the inhibition of tumor growth[14-20].
In the present study, we constructed a target fragment that contained a sequence encoding a secretory signal (SS) and a preactivation peptide (PA), the N-terminal kringle1-4 sequence of plasminogen and 11 amino acids in the C-terminal HA-tagged gene. The target gene fragment of 1.4 kb in length was cloned into Xba I and Hind III restriction sites of eukaryotic expression vector pcDNA3.1 (+). Restriction digestion and sequencing analysis for positive clones indicated that a recombinant eukaryotic expression vector containing the angiostatin gene was successfully constructed. This recombinant vector was then transfected into human liver cancer cell line SMMC-7721 and screened by G418. The result of RT-PCR showed that the recombinant plasmid was stably integrated into the cells. By Western blotting, a protein (Mr = 38000) was detected, indicating that angiostatin protein was expressed in transfected SMMC-7721 cells. Furthermore, the growth curve showed that there was no significant difference in growth rate between transfected and non-transfected SMMC-7721 cells. This indicated that angiostatin had no inhibitory effect on the growth of SMMC-7721 cells.
CD34 is specifically located in endothelial cells. It was reported that CD34 could be regarded as a good marker of endothelial cells in liver cancer because of its high sensibility and specificity. Therefore, the expression of CD34 can reflect the angiogenesis of primary liver cancer and implanted liver tumors. So in this experiment we used CD34 antibody to label endothelial cells.
In this experiment, SMMC-7721 cells that stably expressed angiostatin cDNA (angiostatin group), SMMC-7721 cells alone (blank control group) and SMMC-7721 cells transfected with pcDNA3.1 (+) vector (vector control group) were subcutaneously implanted into nude mice respectively. We found that the implanted tumors appeared later in the angiostatin group, and the mass and volume of the tumor were significantly lower and smaller than those of the other two groups. The inhibitory rate reached 78.6%, and the mass was only 34.6% of that of the blank control group. These results suggested that angiostatin could significantly inhibit the growth of primary tumors. The MVD of the tumor in the angiostatin group was much less than that in the other two groups (P < 0.01). These data indicated that angiostatin could upregulate the expression of angiogenic inhibitory factors and/or downregulate the expression of angiogenic stimulus factors after its cDNA was transfected into SMMC-7721 cells. This would change the balance between angiogenesis stimulus factors and angiogenic inhibitory factors, thus inhibiting of the angiogenesis and growth of tumors.
Gene therapy, concerning the special angiogenic inhibitory factor of endothelial cells of tumor tissue, is a new way of cancer treatment. We believe that this treatment in combination with chemotherapy and radiotherapy would definitely improve the effect of cancer treatment. Because liver cancer is more prevalent in the world[21-23], especially in China[24], our results have the significance in further research.
Edited by Chen WW and Wang XL Proofread by Xu FM
| 1. | Compagni A, Christofori G. Recent advances in research on multistage tumorigenesis. Br J Cancer. 2000;83:1-5. [PubMed] |
| 2. | Detmar M. Tumor angiogenesis. J Investig Dermatol Symp Proc. 2000;5:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Maehara Y, Kabashima A, Koga T, Tokunaga E, Takeuchi H, Kakeji Y, Sugimachi K. Vascular invasion and potential for tumor angiogenesis and metastasis in gastric carcinoma. Surgery. 2000;128:408-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Gervaz P, Scholl B, Mainguene C, Poitry S, Gillet M, Wexner S. Angiogenesis of liver metastases: role of sinusoidal endothelial cells. Dis Colon Rectum. 2000;43:980-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Sabo E, Boltenko A, Sova Y, Stein A, Kleinhaus S, Resnick MB. Microscopic analysis and significance of vascular architectural complexity in renal cell carcinoma. Clin Cancer Res. 2001;7:533-537. [PubMed] |
| 6. | Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6437] [Cited by in RCA: 6487] [Article Influence: 259.5] [Reference Citation Analysis (0)] |
| 7. | Cao Y, O'Reilly MS, Marshall B, Flynn E, Ji RW, Folkman J. Expression of angiostatin cDNA in a murine fibrosarcoma suppresses primary tumor growth and produces long-term dormancy of metastases. J Clin Invest. 1998;101:1055-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 169] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Luo YQ, Wu MC, Cong WM. Gene expression of hepatocyte growth factor and its receptor in HCC and nontumorous liver tissues. World J Gastroenterol. 1999;5:119-121. [PubMed] |
| 9. | Barinaga M. Designing therapies that target tumor blood vessels. Science. 1997;275:482-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2364] [Cited by in RCA: 2266] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 11. | Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350-4354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33019] [Cited by in RCA: 36592] [Article Influence: 795.5] [Reference Citation Analysis (0)] |
| 12. | Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4848] [Cited by in RCA: 4795] [Article Influence: 165.3] [Reference Citation Analysis (0)] |
| 13. | Araya M, Terashima M, Takagane A, Abe K, Nishizuka S, Yonezawa H, Irinoda T, Nakaya T, Saito K. Microvessel count predicts metastasis and prognosis in patients with gastric cancer. J Surg Oncol. 1997;65:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Cao Y, Moses M, Lane WS, Sage EH, Folkman J. Angiostatin: a circulating endothelial cell inhibitor that suppresses angiogenesis and tumor growth. Cold Spring Harb Symp Quant Biol. 1994;59:471-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Stack MS, Gately S, Bafetti LM, Enghild JJ, Soff GA. Angiostatin inhibits endothelial and melanoma cellular invasion by blocking matrix-enhanced plasminogen activation. Biochem J. 1999;340:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Hari D, Beckett MA, Sukhatme VP, Dhanabal M, Nodzenski E, Lu H, Mauceri HJ, Kufe DW, Weichselbaum RR. Angiostatin induces mitotic cell death of proliferating endothelial cells. Mol Cell Biol Res Commun. 2000;3:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Ijland SA, Jager MJ, Heijdra BM, Westphal JR, Peek R. Expression of angiogenic and immunosuppressive factors by uveal melanoma cell lines. Melanoma Res. 1999;9:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Matsuda KM, Madoiwa S, Hasumi Y, Kanazawa T, Saga Y, Kume A, Mano H, Ozawa K, Matsuda M. A novel strategy for the tumor angiogenesis-targeted gene therapy: generation of angiostatin from endogenous plasminogen by protease gene transfer. Cancer Gene Ther. 2000;7:589-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Volm M, Mattern J, Koomägi R. Angiostatin expression in non-small cell lung cancer. Clin Cancer Res. 2000;6:3236-3240. [PubMed] |
| 20. | Wu J, Shi YQ, Wu KC, Zhang DX, Yang JH, Fan DM. Angiostatin up-regulation in gastric cancer cell SGC7901 inhibits tumorigenesis in nude mice. World J Gastroenterol. 2003;9:59-64. [PubMed] |
| 21. | El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 684] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 22. | Regimbeau JM, Abdalla EK, Vauthey JN, Lauwers GY, Durand F, Nagorney DM, Ikai I, Yamaoka Y, Belghiti J. Risk factors for early death due to recurrence after liver resection for hepatocellular carcinoma: results of a multicenter study. J Surg Oncol. 2004;85:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Ku Y, Iwasaki T, Tominaga M, Fukumoto T, Takahashi T, Kido M, Ogata S, Takahashi M, Kuroda Y, Matsumoto S. Reductive surgery plus percutaneous isolated hepatic perfusion for multiple advanced hepatocellular carcinoma. Ann Surg. 2004;239:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Lin GY, Chen ZL, Lu CM, Li Y, Ping XJ, Huang R. Immunohistochemical study on p53, H-rasp21, c-erbB-2 protein and PCNA expression in HCC tissues of Han and minority ethnic patients. World J Gastroenterol. 2000;6:234-238. [PubMed] |