Published online May 15, 2004. doi: 10.3748/wjg.v10.i10.1397
Revised: December 9, 2003
Accepted: December 16, 2003
Published online: May 15, 2004
AIM: Hepatocellular carcinoma (HCC) with bile duct tumor thrombosis (BDT) is a rare event. The prognosis of this type of patients is very dismal. The aim of this study was to share the experience in the diagnosis and treatment of HCC with BDT, to further improve the prognosis of these patients.
METHODS: Thirty-four patients of HCC with BDT received surgical treatment in authors’institute from July 1987 to January 2003 were reviewed retrospectively. The experience in the diagnosis and treatment, and the outcome of this type of HCC patients were summarized.
RESULTS: Thirty of the 34 patients (88.2%) were positive for alpha-fetoprotein (AFP) (> 20 μg/L), and 12 patients (35.3%) were found having obstructive jaundice before operation, 18 cases were suspected of “obstruction of bile duct” preoperatively. The primary tumors were frequently located at the left medial (13 cases) or right anterior lobe (14 cases). Thirty-one patients received liver resections and removal of BDT, while the other 3 patients received removal of BDT combined with hepatic artery ligation and cannulation (HAL + HAI), or only removal of BDT because their liver function reservation and general condition could not tolerate the primary tumor resection. The 1-year survival rate was 71.4% (20/28). The longest disease-free survival was over 15 years. The intrahepatic tumor recurrence within 1 year after operation was found in 14 patients (14/28, 50.0%).
CONCLUSION: Surgical removal of primary tumors and BDT is safe and beneficial to the HCC patients with BDT. Early detection, diagnosis, and surgical treatment are the key points to prolong the survival time of patients.
- Citation: Qin LX, Ma ZC, Wu ZQ, Fan J, Zhou XD, Sun HC, Ye QH, Wang L, Tang ZY. Diagnosis and surgical treatments of hepatocellular carcinoma with tumor thrombosis in bile duct: Experience of 34 patients. World J Gastroenterol 2004; 10(10): 1397-1401
- URL: https://www.wjgnet.com/1007-9327/full/v10/i10/1397.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i10.1397
Obstructive jaundice as the main presenting clinical feature is uncommon in patients with hepatocellular carcinoma (HCC). Only 1%-12% of patients with HCC manifest obstructive jaundice as the initial complaint[1-3]. Mallory et al[4] described the first such case in 1947, in which HCC invaded the cystic duct and gave rise to obstructive jaundice caused by the tumor thrombosis. These kinds of patients were clinically classified as “icteric-type hepatoma”[5] or “cholestatic type of HCC”[6].
Tumor thrombus in bile duct (BDT) is one of the main reasons for obstructive jaundice. The incidence was 1.2%-9% in previous reports[2,3,5-8]. However, Huang et al[9] found the incidence was only 0.53%. It is usually difficult to make diagnosis before operation, because of the low incidence rate, ignorance of this disease, and the difficulty for the imaging diagnosis to find the BDT preoperatively. The prognosis of this type of HCC patients is very dismal, but is better than those with jaundice caused by hepatic insufficiency. Identification of this group of patients is clinically important, because surgical treatment may be beneficial. In this study, we summarized our experience in the diagnosis and treatment of 34 cases of this type of HCC during the past 15 years.
From July 1987 to January 2003, totally 4324 patients suffering from HCC received surgical treatment in Liver Cancer Institute and Zhongshan Hospital, Fudan University (former Shanghai Medical University), and 34 cases (0.79%) were found having tumor thrombosis in bile duct. Among of them, 28 cases were male, and 6 cases were female. The mean age of patients was 48.5 years (32-76 years). The history of hepatitis B virus (HBV) infection or HBsAg positive was found in all of the patients, and liver cirrhosis in 94.1% (32/34) of patients. Thirty of them (30/34, 88.2%) were positive for alpha-fetoprotein (AFP) (> 20 μg/L), and the highest was over 2000 μg/L. Preoperative obstructive jaundice was found in 12 patients (12/34, 35.3%), and 2 of them had the history of “transient cholangitis” with the manifestation of transient jaundice. The history of “hemorrhage of upper digestive system” was found in 2 patients. Four patients had the history of preoperative transcatheter arterial chemoembolization (TACE) (Table 1).
| Items | Cases (%) |
| Sex | |
| Male | 28 (82.4%) |
| Female | 6 |
| HBV infection | |
| HBsAg+ | 34 (100%) |
| Liver cirrhosis | 32 (94.1%) |
| AFP | |
| > 20 μg/L | 30 (88.2%) |
| ≤ 20 μg/L | 4 |
| Obstructive jaundice | |
| + | 12 (12, 35.3%) |
| - | |
| Ueda classification | |
| Type I | 2 |
| Type II | 8 |
| Type IIIa | 16 |
| Type IIIb | 1 |
| Type IV | 7 |
| Treatment | |
| Surgical resection | 31 (91.2%) |
| Removal of BDT + HAL + HAI | 2 |
| Only removal of BDT | 1 |
| Survival | |
| > 1 yr | 20 (20/28, 71.4%) |
| > 3 yr | 3 |
| > 15 yr | 1 |
| HCC recurrence | |
| Within 1 yr | 14 (14/28, 50.0%) |
| Within 3 mo | 9 (9/31, 29.0%) |
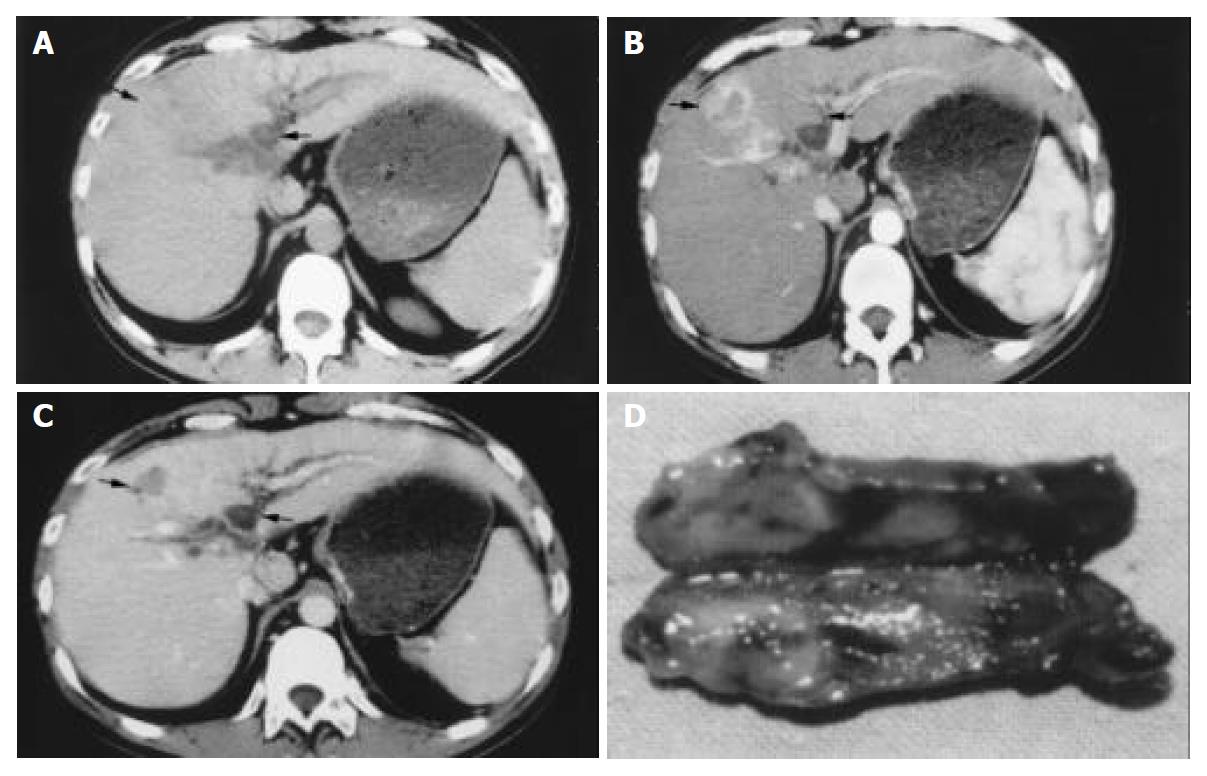
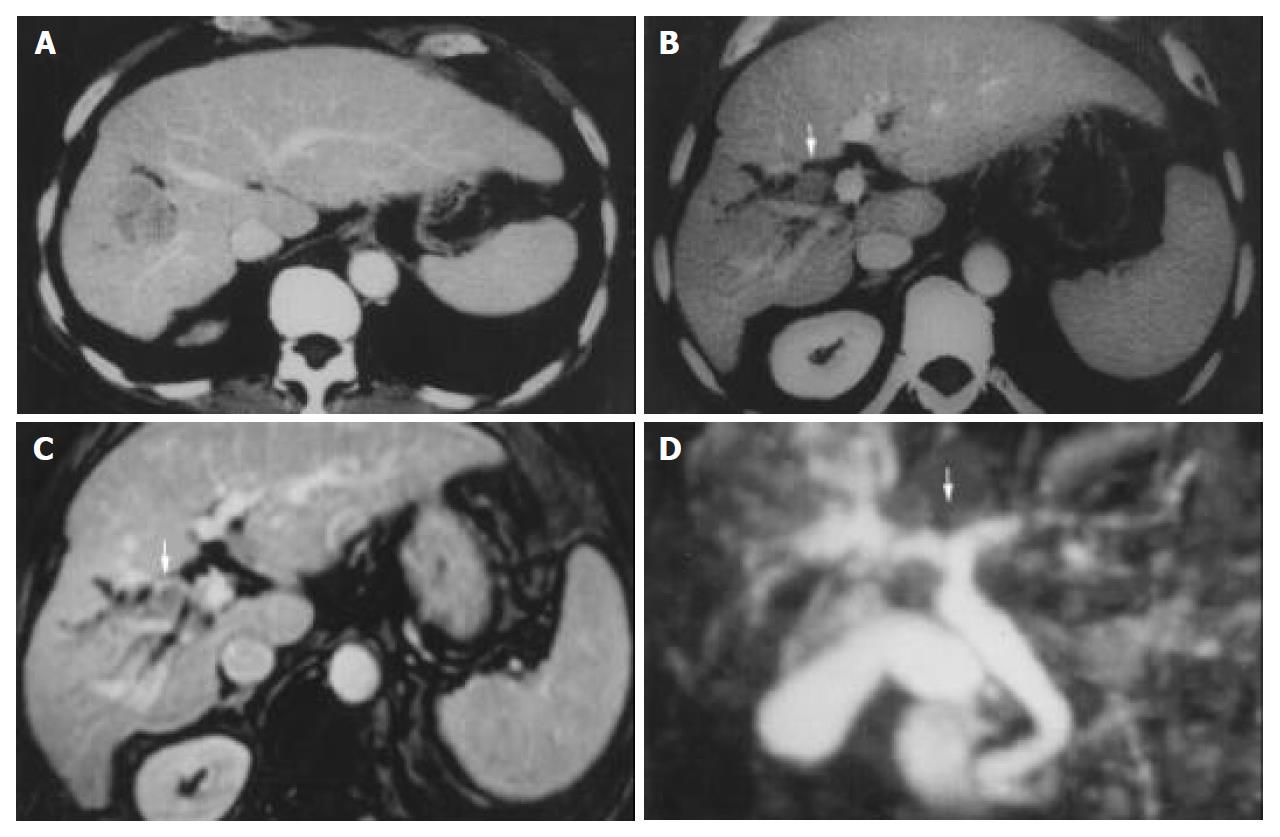
Ultrasonography (US) and CT scan were performed in all of the patients. Magnetic resonance cholangiography (MRCP) was also done in 12 patients in recent 3 years. (Figure 1, Figure 2) Eighteen cases were suspected of “obstruction of bile duct” because of the occurrence of preoperative jaundice (12 cases), and/or dilation of intrahepatic bile duct shown in imaging diagnosis (in 6 cases without obstructive jaundice). Only 9 cases of them were obviously shown tumor thrombosis in the bile duct by US, CT scan or MRI preoperatively. One case with neoplasm in the bile duct, while no obvious intrahepatic lesion, was misdiagnosed as cholangiocarcinoma, in spite of the positive AFP.
The size of primary tumors was 6.4 ± 2.5 cm in diameter (2-15 cm). All of the primary tumors had no capsule, with unclear tumor margin, and invasive pattern of growth. The primary tumors were located at the segment IV in 13 cases, right anterior section (segments V and VIII) in 14 cases, segment I (caudate lobe) in 1 case, segment II-III in 4 cases, and segment VI in 2 cases. The tumor thrombus located at left hepatic duct (LHD) in 5 cases, LHD to common hepatic duct (CHD)/common bile duct (CBD) in 9 cases, right hepatic bile duct (RHD) in 5 cases, RHD to CHD/CBD in 8 cases, and CBD in 7 cases. According to Ueda classification[10], 2 cases belonged to type I, 8 cases type II, 16 cases type IIIa, 1 case type IIIb, and 7 cases type IV. In 2 cases, the BDT was as long as 6 cm, and 9 cm, respectively (Table 1, Figure 1).
All of the patients received surgical treatment. Thirty-one patients received liver resection and removal of the tumor thrombosis (or thrombectomy). Among them, 12 patients received left hemihepatectomy, 2 cases received left lateral sectionectomy, 11 cases received limited partial resection of the liver, 5 cases received right hemihepatectomy, and 1 case received resection of the left lobe and caudate lobe of liver, and CHD, and RHD-jejunum anastomosis. Two patients received removal of CBD thrombosis combined with hepatic artery ligation and cannulation (HAL + HAI), and 1 patient received removal of BDT only because their liver function reservation and general condition could not tolerate the primary tumor resection.
The tumor thrombi were removed by the exploration of CBD in 14 cases, from the cut end of the bile duct after liver resection in 15 cases, and en bloc removal with the primary tumor in 5 patients. Intraoperative active bleeding from the CHD after removal of the thrombosis happened in 1 patient, and hemostasis was achieved finally by infusing noradrenalin in normal saline into the bile duct and oppressing locally.
The operations were well tolerated. After operation, the obstructive jaundice due to BDT was successfully relieved in all but 2 patients. One patient with severe liver cirrhosis and preoperative obstructive jaundice (the total serum bilirubin was 182 μmol/L) received partial resection of the right liver lobe, and died of liver failure at the 35th d after the operation. Another patient with severe preoperative obstructive jaundice (total serum bilirubin was 320 μmol/L, and the direct bilirubin was 210 μmol/L) received right hemihepatectomy and removal of thrombosis in CBD, the total serum bilirubin did not decrease although the general condition of the patient was good, the patient himself chose to give up the treatment and left the hospital at the 40th d after the operation. A third patient with obstructive jaundice (preoperative total serum bilirubin was 156 μmol/L) due to BDT in the CBD received left hemihepatectomy and removal of BDT. His total serum bilirubin rose to over 700 μmol/L in 2 wk after operation, and finally returned to normal in 3 mo. By now, this patient has survived for 11 mo.
The follow-up was up to February of 2003. Twenty-eight patients were followed-up over 1 year. Twenty patients survived over 1 year. One-year survival rate was 71.4% (20/28). The longest disease-free survival time was over 15 years. It occurred in one female patient who received left hemihepatectomy and removal of the tumor thrombosis in CBD (Ueda type IV) in July of 1987. She was still alive without recurrence of cancer up to the last follow-up. Another female patient who received partial resection of right lobe and removal of BDT from the RHD (Ueda type II) in August of 1993 had survived over 9 years without recurrence. The third female patient received right hemihepatectomy and removal of CHD thrombosis (Ueda type IIIa) in May of 1995, and received the second operation due to HCC recurrence 3.5 years later. One male patient who received right hemihepatectomy in September of 1999 (Type II) had also survived over 2 years without recurrence. Fourteen patients (14/28, 50.0%) were found intrahepatic HCC recurrence within 1 year after operation. Nine of them (9/31, 29.0%) were found intrahepatic recurrence within 3 mo after operations. The survival times of the 3 patients received biliary decompression were only 2, 3, and 3.5 mo, respectively (Table 1).
Obstructive jaundice caused by BDT, especially as the initial presentation of HCC, is a rare event. And little is known about this type of HCC. However, there are more and more reports about this type of disease, and the incidence is increasing in patients with HCC[11]. In this study, only 34 out of the 4324 HCC patients who received surgical treatment in authors’ institute were found BDT. The incidence was only 0.79%. And more, most of them (26/34, 76.5%) were treated in recent 5 years. This might be due to the increased understanding of this type of HCC, further improvement of diagnosis (particularly the imaging diagnosis), and more positive attitude towards the treatment to this kind of patients.
HCC invades into bile duct through the following different mechanisms: a distal tumor may grow continuously until it fills the entire extrahepatic biliary system; a fragment of necrotic tumor may separate from the proximal intraductal growth, migrate to the distal common bile duct and cause an obstruction; hemorrhage from the tumor may partially or completely fill the biliary tract with tumor-containing blood clots[2,3,7-9,12,13]. These produce different types of BDT which will also affect the patients’ prognosis[10]. In this series, 4 patients without any obvious evidence of BDT at initial diagnosis were found BDT after TACE treatment, which suggested that TACE might increase the possibility of BDT[11]. The exact mechanism is not clear yet.
Just as other types of HCC, no specific symptoms could be found in the early stage. Only when intraductal tumor growth occurs in the CHD and/or CBD does obstructive jaundice become a clinical concern. The common clinical features include high level of serum AFP, the history of cholangitis with dilation of intrahepatic bile duct, aggravating jaundice and rapidly developing into liver dysfunction. Unexplained hemobilia could be the initial complaint without any manifestation of primary tumor. And “hemorrhage of upper digestive system”, which might be due to the hemorrhage of bile duct (hemobilia), could also be the first manifestation. The most important point is that we should think about the possibility of BDT when these manifestations are found in HCC patients.
There are still difficult and challenging problems in differential diagnosis of this type of HCC before operation. Despite remarkable recent improvements in the imaging techniques for diagnosis of HCC, such cases were still incorrectly diagnosed as cholangiocarcinoma or choledocholithiases[2,7-14]. In this series, only 9 out of the 18 cases suspected of “obstruction of bile duct” were obviously shown BDT with US or CT scan preoperatively. The others were misdiagnosed as “bile duct stone” or cholangiocarcinoma, even though “neoplasm in CBD” was found. All of these suggest that deeper understanding of this type of disease is the key to further improving the diagnosis preoperatively. Further advanced imaging examination should be performed if the intrahepatic bile duct of one lobe or around the tumor is found dilated. The existence of tumor thrombosis in bile duct should be considered when an occupying lesion is found within bile duct. Fortunately, in recent years, more correct preoperative diagnosis could be made in this type of patients.
MR cholangiography (MRCP) is an absolutely noninvasive imaging modality, which has been shown to be superior to ERCP in detecting the presence of biliary obstruction. Both primary liver tumors and dilatation of biliary system could be demonstrated in MRCP[15]. Presence of intraluminal soft tissue at the bile duct, and enhancement of the intraluminal soft tissue in the arterial phase are 2 typical features of HCC with BDT (Figure 2). However, these characteristic cholangiographic features should be differentially diagnosed with papillary type cholangiocarcinoma, intraductal polyps or mucin-hypersecreting intrahepatic biliary neoplasm, or intrahepatic cholangiocarcinoma with invasion of extrahepatic bile duct, and even the bile duct stones[16]. It still relies on other information, such as the presence of liver cirrhosis, hepatitis markers, tumor markers (AFP, CEA), the fluctuation of jaundice, and hemobilia.
The prognosis of this type of HCC is generally dismal, particularly for those with obstructive jaundice[2,3]. However, the prognosis of these patients is better than those HCC patients who have jaundice caused by hepatic insufficiency, which is closely related to the stage of disease, the location and extent of BDT. Different therapies also influence the prognosis of this type of patients. Surgical resection is the only way that possibly cures the patients. Jaundice is not necessarily a harbinger of advanced disease and a contraindication for surgery. The goals of operative intervention are biliary decompression with removal of tumor debris or tumor-containing blood clots, and, if possible, curative resection of the hepatic tumor. The usually used operative methods are lobectomy (including the primary tumor and the tumor thrombosis in bile duct), hepatectomy combined with thrombectomy, biliary decompression and drainage (choledochotomy with T-tube drainage alone, internal biliary stenting, or biliary diversion). The ideal treatment is hepatic resections[17-19]. Patients who received curative liver resection had a much better survival rate than those without resection[20-24]. In our series, the postoperative 1-year survival rate of patients was 71.4%, 1-year disease-free survival rate was 21.4%, and one patient has survived over 15 years. These are better than that of previous reports. It might be attributed to active attitude of the doctors and appropriate procedures of treatment taken. All of the 4 long-term survivors received major liver resection (hemihepatectomy) and removal of BDT, while the 3 patients who received biliary decompression only survived 2, 3, and 3.5 mo, respectively. So, to improve survival, if the patient’s liver function and general condition could tolerate, it is suggested to perform major liver resection with removal of BDT. However, it should be very careful to perform major liver resection for those patients with both severe liver cirrhosis and severe obstructive jaundice, because their liver function reservation is very poor. In this series, one patient died of liver failure postoperatively even though his liver function test was good (except for obstructive jaundice). If hepatic resection cannot be accomplished with bile duct resection due to limited liver function, non-surgical modalities should be considered instead of surgery.
Surgical intervention is very effective to relieve the obstruction of bile duct in this type of patients. It could take a long time (such as 3 mo in one case of this study) for the serum bilirubin of patients with obstructive jaundice to return to normal, or even transiently increase in short time after operation though the obstruction in bile duct has been dispelled completely. The altering pattern and the duration for the serum bilirubin to return to normal after operation, especially for those with sever preoperative obstructive jaundice, still need further studying.
The ideal way to remove BDT is en bloc resection with the primary tumor. It is also relatively easy to remove BDT either with the exploration of CBD or from the cut-end of bile duct after hepatectomy. However, active hemorrhage occurred during operation in some cases, possibly because of the continuity of the intraductal tumor debris with the main intrahepatic tumor. In this study, we met one patient with active bleeding during the operation after removing the thrombosis from the common hepatic duct. The hemostasis was achieved finally by infusing noradrenalin in normal saline into the bile duct and oppressing locally. Suturing, electrocauterization, compression, Pringle’s maneuver, or hepatic arterial ligation are some alternative ways to achieve hemostasis.
BDT often grows faster than the primary cancer. We found in 2 cases, the BDT was as long as 6 cm, and 9 cm, respectively, while their primary HCC less than 6 cm. The primary tumor often has no capsule, with unclear tumor margin, and invasive growth. The infiltrative nature of this particular type of HCC may in part explain their invasion of the biliary tree early in their growth without regard to tumor size or type[25,26], which might also be one of the reasons that the prognosis of this type of patients was very poor. In this series, the 1-year recurrence rate was 50.0%, and as high as 29.0% of the patients were found having tumor recurrence within 3 mo after operation. This might also indicate the poor malignant phenotype of this type of HCC. Combined chemotherapy or chemoembolization might be helpful to control the postoperative recurrence[27].
In summary, HCC with tumor thrombosis in bile duct, particularly with obstructive jaundice as the main presenting clinical feature, is uncommon. The prognosis of this type of HCC is generally dismal, but is better than those HCC patients who have jaundice caused by hepatic insufficiency. Jaundice is not necessarily a harbinger of advanced disease and a contraindication for surgery. If appropriate procedures are selected and carried out safely, it can result in long-term relief of symptoms and occasional long-term survival. Deeper understanding, and active attitude to treatment of this type of disease are the keys to further improving survival of these patients.
Edited by Zhu LH Proofread by Xu FM
| 1. | Kew MC, Paterson AC. Unusual clinical presentations of hepatocellular carcinoma. Trop Gastroenterol. 1985;6:10-22. [PubMed] |
| 2. | Kojiro M, Kawabata K, Kawano Y, Shirai F, Takemoto N, Nakashima T. Hepatocellular carcinoma presenting as intrabile duct tumor growth: a clinicopathologic study of 24 cases. Cancer. 1982;49:2144-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | Qin LX, Tang ZY. Hepatocellular carcinoma with obstructive jaundice: diagnosis, treatment and prognosis. World J Gastroenterol. 2003;9:385-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 109] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Mallory TB, Castleman B, Parris EE. Case records of the Massa-chusetts General Hospital. N Eng J Med. 1947;237:673-676. |
| 5. | Lin TY, Chen KM, Chen YR, Lin WS, Wang TH, Sung JL. Icteric type hepatoma. Med Chir Dig. 1975;4:267-270. [PubMed] |
| 6. | Okuda K. Clinical aspects of hepatocellular carcinoma: analysis of 134 cases. Hepatocellular carcinoma. New York: John Wiley 1976; 387-436. |
| 7. | Jan YY, Chen MF. Obstructive jaundice secondary to hepatocellular carcinoma rupture into the common bile duct: choledochoscopic findings. Hepatogastroenterology. 1999;46:157-161. [PubMed] |
| 8. | Lau WY, Leung JW, Li AK. Management of hepatocellular carcinoma presenting as obstructive jaundice. Am J Surg. 1990;160:280-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Huang JF, Wang LY, Lin ZY, Chen SC, Hsieh MY, Chuang WL, Yu MY, Lu SN, Wang JH, Yeung KW. Incidence and clinical outcome of icteric type hepatocellular carcinoma. J Gastroenterol Hepatol. 2002;17:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Ueda M, Takeuchi T, Takayasu T, Takahashi K, Okamoto S, Tanaka A, Morimoto T, Mori K, Yamaoka Y. Classification and surgical treatment of hepatocellular carcinoma (HCC) with bile duct thrombi. Hepatogastroenterology. 1994;41:349-354. [PubMed] |
| 11. | Spahr L, Frossard JL, Felley C, Brundler MA, Majno PE, Hadengue A. Biliary migration of hepatocellular carcinoma frag-ment after transcatheter arterial chemoembolization therapy. Eur J Gastroenterol Hepatol. 2000;12:243-244. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Afroudakis A, Bhuta SM, Ranganath KA, Kaplowitz N. Obstructive jaundice caused by hepatocellular carcinoma. Report of three cases. Am J Dig Dis. 1978;23:609-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Buckmaster MJ, Schwartz RW, Carnahan GE, Strodel WE. Hepatocellular carcinoma embolus to the common hepatic duct with no detectable primary hepatic tumor. Am Surg. 1994;60:699-702. [PubMed] |
| 14. | Wang JH, Chen TM, Tung HD, Lee CM, Changchien CS, Lu SN. Color Doppler sonography of bile duct tumor thrombi in hepatocellular carcinoma. J Ultrasound Med. 2002;21:767-772; quiz 773-774. [PubMed] |
| 15. | Fulcher AS, Turner MA, Capps GW, Zfass AM, Baker KM. Half-Fourier RARE MR cholangiopancreatography: experience in 300 subjects. Radiology. 1998;207:21-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 215] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Yeh TS, Jan YY, Tseng JH, Chiu CT, Chen TC, Hwang TL, Chen MF. Malignant perihilar biliary obstruction: magnetic resonance cholangiopancreatographic findings. Am J Gastroenterol. 2000;95:432-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Chen MF, Jan YY, Jeng LB, Hwang TL, Wang CS, Chen SC. Obstructive jaundice secondary to ruptured hepatocellular carcinoma into the common bile duct. Surgical experiences of 20 cases. Cancer. 1994;73:1335-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Jan YY, Chen MF, Chen TJ. Long term survival after obstruction of the common bile duct by ductal hepatocellular carcinoma. Eur J Surg. 1995;161:771-774. [PubMed] |
| 19. | Tantawi B, Cherqui D, Tran van Nhieu J, Kracht M, Fagniez PL. Surgery for biliary obstruction by tumour thrombus in primary liver cancer. Br J Surg. 1996;83:1522-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Lau W, Leung K, Leung TW, Liew CT, Chan MS, Yu SC, Li AK. A logical approach to hepatocellular carcinoma presenting with jaundice. Ann Surg. 1997;225:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Wang HJ, Kim JH, Kim JH, Kim WH, Kim MW. Hepatocellular carcinoma with tumor thrombi in the bile duct. Hepatogastroenterology. 1999;46:2495-2499. [PubMed] |
| 22. | Hu J, Pi Z, Yu MY, Li Y, Xiong S. Obstructive jaundice caused by tumor emboli from hepatocellular carcinoma. Am Surg. 1999;65:406-410. [PubMed] |
| 23. | Lau WY, Leung KL, Leung TW, Ho S, Chan M, Liew CK, Leung N, Johnson P, Li AK. Obstructive jaundice secondary to hepatocellular carcinoma. Surg Oncol. 1995;4:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Nishio H, Miyata K, Hanai M, Kato M, Yoneyama F, Kobayashi Y. Resection of an icteric type hepatoma with tumor thrombi filling the right posterior bile duct. Hepatogastroenterology. 2002;49:1682-1685. [PubMed] |
| 25. | Huang GT, Sheu JC, Lee HS, Lai MY, Wang TH, Chen DS. Icteric type hepatocellular carcinoma: revisited 20 years later. J Gastroenterol. 1998;33:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Tseng JH, Hung CF, Ng KK, Wan YL, Yeh TS, Chiu CT. Icteric-type hepatoma: magnetic resonance imaging and magnetic resonance cholangiographic features. Abdom Imaging. 2001;26:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Fukuda S, Okuda K, Imamura M, Imamura I, Eriguchi N, Aoyagi S. Surgical resection combined with chemotherapy for advanced hepatocellular carcinoma with tumor thrombus: report of 19 cases. Surgery. 2002;131:300-310. [RCA] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |