Published online May 15, 2004. doi: 10.3748/wjg.v10.i10.1392
Revised: December 1, 2003
Accepted: December 8, 2003
Published online: May 15, 2004
AIM: To investigate the effect of c9,t11-conjugated linoleic acid (c9, t11-CLA) on the adhesion of human gastric carcinoma cell line (SGC-7901).
METHODS: SGC-7901 cells were at first treated with different concentrations (25, 50, 100, 200 μmol/L) of c9, t11-CLA and 1 mL/L ethanol (as a negative control) for 24 h. Using adhesion assay and Western blot, we investigated the ability of SGC-7901 cells to adhere to intracellular matrix and examined the expression of E-cadherin (ECD), α-catenin, intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in these cells.
RESULTS: The attachment rate to laminin of SGC-7901 cells treated with different concentrations of c9, t11-CLA (0, 25, 50, 100, and 200 μmol/L) was 100.0 ± 3.3, 95.7 ± 4.0, 89.2 ± 4.6, 87.9 ± 6.1, and 65.9 ± 5.8, respectively. The attachment rate to fibronectin was 100.0 ± 4.7, 96.8 ± 3.8, 94.5 ± 4.1, 76.5 ± 4.3, and 61.8 ± 4.8, respectively. The attachment rate to Matrigel was 99.9 ± 6.6, 91.4 ± 6.8, 85.5 ± 7.4, 79.3 ± 5.6, and 69.6 ± 5.1, respectively. Besides, c9, t11-CLA could increase the level of ECD and α-catenin, and decrease the level of ICAM-1 and VCAM-1 in SGC-7901 cells.
CONCLUSION: c9,t11-CLA can reduce the adhesion of human gastric carcinoma cells to laminin, fibronectin and Matrigel. c9,t11-CLA can increase the level of ECD and α-catenin, and decrease the level of ICAM-1 and VCAM-1 in human gastric carcinoma cells.
-
Citation: Chen BQ, Yang YM, Wang Q, Gao YH, Liu JR, Zhang JS, Wang XL, Liu RH. Effects of
c 9,t 11-conjugated linoleic acid on adhesion of human gastric carcinoma cell line SGC-7901. World J Gastroenterol 2004; 10(10): 1392-1396 - URL: https://www.wjgnet.com/1007-9327/full/v10/i10/1392.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i10.1392
Although the incidence of gastric cancer is decreasing worldwide, it remains one of the most common tumors in China[1-4] and is a major cause of cancer deaths in some countries[5,6]. Most of gastric cancer patients die from metastasis. Although the mechanism of gastric cancer metastasis is not fully elucidated, the abnormal adhesion ability of cells has been reported to play a pivotal role. Cell-cell and cell-matrix adhesions are essential for establishing and maintaining normal cell morphology and function. Disturbance of cell adhesion may result in the malignant transformation of cells. Furthermore, cell adhesion molecules are important ingredients in maintaining cell-cell adhesion and cell-matrix interactions. The abnormality of cell adhesion molecules closely correlates with neoplastic transformation and metastasis[7,8]. Cell adhesion molecules mediate tumor cell-cell, tumor cell-endothelial cell and tumor cell-matrix interactions. In tumor metastasis, cell-cell and cell-matrix interactions are determined by functional status of cell adhesion molecules. Glycoproteins are the cell adhesion molecules and can be classified into several classes according to their structure: cadherins, selectins, CD44, immunoglobulin family, and integrin family.
Conjugated linoleic acid (CLA) is a class of positional and stereoisomers of octadecadienoate (18:2) with conjugated double bonds. The predominant isomer in foods is the c9,t11-CLA isomer[9-16]. In 1979, Pariza et al[17] detected mutagenic inhibitory activity in both cooked and uncooked ground beef. Then in 1985, they observed that the crude extracts could protect rats against tumors[18]. In 1987, Ha et al[19] identified four isomers of CLA from cooked beef. In several animal models of chemical carcinogenesis, it has been reported that CLA was a potent cancer preventive agent. For example, CLA could inhibit skin papillomas[18,20], forestomach neoplasia[21-23], mammary tumors[24-30], and colon aberrant crypt foci[31]. Moreover, CLA was also effective in reducing the size and metastasis of transplanted human breast cancer cells and prostate cancer cells in SCID mice[32,33]. Several studies[34-43] suggested that CLA was cytostatic and cytotoxic to a variety of human cancer cells in vitro, including hepatoma, malignant melanoma, colorectal cancer, breast carcinoma, and gastric cancer.
One of our previous studies showed that c9,t11-CLA could inhibit the invasion of mouse melanoma cells (B16-MB) through reducing their adhesion ability to extracellular matrix[44]. Two other studies of ours showed that c9,t11-CLA could decrease the invasive ability of human gastric carcinoma cells (SGC-7901)[45,46]. However, it is unclear whether CLA influences the adhesive ability of SGC-7901 cells and the expression of their adhesion molecules. Therefore, in this study, we investigated the effect of c9,t11-CLA on the adhesive ability of SGC-7901 cells and detected the expression of E-cadherin (ECD), α-catenin, intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in SGC-7901 cells using adhesion and Western blot assays.
c9,t11-CLA with 98% purity, was provided by Dr. Rui-Hai Liu at Food Science and Toxicology, Department of Food Science, Cornell University, Ithaca, NY, USA. The c9,t11-CLA was dissolved in ethanol, then diluted to the following concentrations: 25, 50, 100, and 200 μmol/L.
To examine the expression of ECD, α-catenin, ICAM-1, and VCAM-1, we used four primary antibodies: rabbit polyclonal antibody for ECD, mouse monoclonal antibody for α-catenin, and goat polyclonal antibodies for ICAM-1 and VCAM-1, respectively. These antibodies were purchased from Zhongshan Co., China.
Cell culture Human gastric adenocarcinoma cells (SGC-7901), purchased from Cancer Research Institute of Beijing (China), were cultured in RPMI 1640 (Gibco) medium, supplemented with 100 mL/L fetal calf serum (FCS), 100 × 103 U/L penicillin, 100 mg/L streptomycin and 2 mmol/L L-glutamine under 50 mL/L CO2 in a humidified incubator. The pH was maintained at 7.2-7.4 and the temperature at 37 °C. After sub-cultured with EDTA, the SGC-7901 cells were incubated with different concentrations (25, 50, 100, and 200 μmol/L) of c9,t11-CLA and 1ml/L ethanol (as a negative control) for 24 h.
Cell adhesion assay A total of 96-well plates (Nunc. Co.) were incubated at 37 °C with laminin, fibronectin or Matrigel for 1 h and then blocked with phosphate-buffered saline (PBS) containing 100 g/L BSA at the same temperature for another 1 h. After exposed to different concentrations (25, 50, 100, and 200 μmol/L) of c9,t11-CLA for 24 h, the SGC-7901 cells were suspended in serum-free medium at a density of 8 × 105 cells/ml. Then, 0.1 mL of SGC-7901 cells suspension was added to each well and incubated at 37 °C for 1 h. The plates were washed three times with PBS to remove unattached cells. The remaining SGC-7901 cells in 96-well plates were reacted with MTT for 4 h at 37 °C, then solved with DMSO. The absorbance of each well was measured at 570 nm with an ELX800 microplate reader (Bio-TEK Co.). Results were expressed as the percentage of total cells assuming that the adhesion of cells in control was 100%.
Protein extract and western blot The SGC-7901 cells treated with different concentrations of c9,t11-CLA were harvested, washed twice times with PBS and lysed at 4 °C in lysis buffer containing 150 mmol/L NaCl, 1 mL/L NP-40, 5 mg/L sodium deoxycholate, 100 g/L SDS, 50 mmol Tris (pH7.4), 1 mmol/L DTT, 0.5 mmol/L Na3VO4, 10 mmol/L phenylmethylsulfonyl fluoride (PMSF), 10 mg/L aprotinin, and 5 mg/L leupeptin. Following the centrifugation of 10000 g for 30 min at 4 °C, the amount of protein in the supernatant was determined using DUR 640 nucleic acid and protein analyzer. Equal amount of protein was separated on SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane (Gibco BRL, USA) overnight. Blocked with 50 g/L defatted milk, the membrane was hybridized with rabbit anti-E-cadherin, mouse anti-α-catenin, goat anti-ICAM-1 and goat anti-VCAM-1 antibody, then incubated with horseradish peroxidase- conjugated IgG. Finally, the immunoreactive bands were detected using diaminobenzidine tetrahydrochloride (DAB) substrate and analyzed with a ChemiImagerTM 4000 low light imaging system (Alpha Innotech Corporation). At the same time GAPDH was used as house-keeping protein.
As shown in Table 1, c9,t11-CLA could reduce the cell attachment to FN, LN or Matrigel in a dose dependent manner after SGC-7901 cells were pre-incubated for 24 h with different concentrations of c9,t11-CLA.
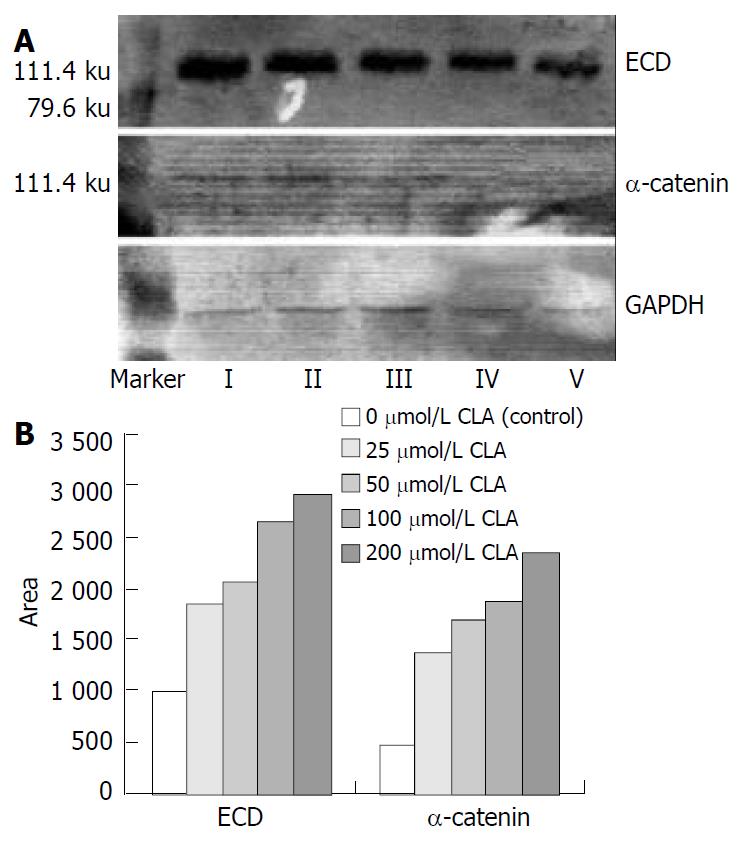
As shown in Figure 1, the level of ECD and α-catenin protein in SGC-7901 cells treated with different concentrations of c9,t11-CLA was increased in comparison with that in the negative control group. The level of ECD and α-catenin protein in SGC-7901 cells reated with 200 μmol/L c9,t11-CLA increased 65.9% and 80.5% respectively, compared with those in the negative control group.
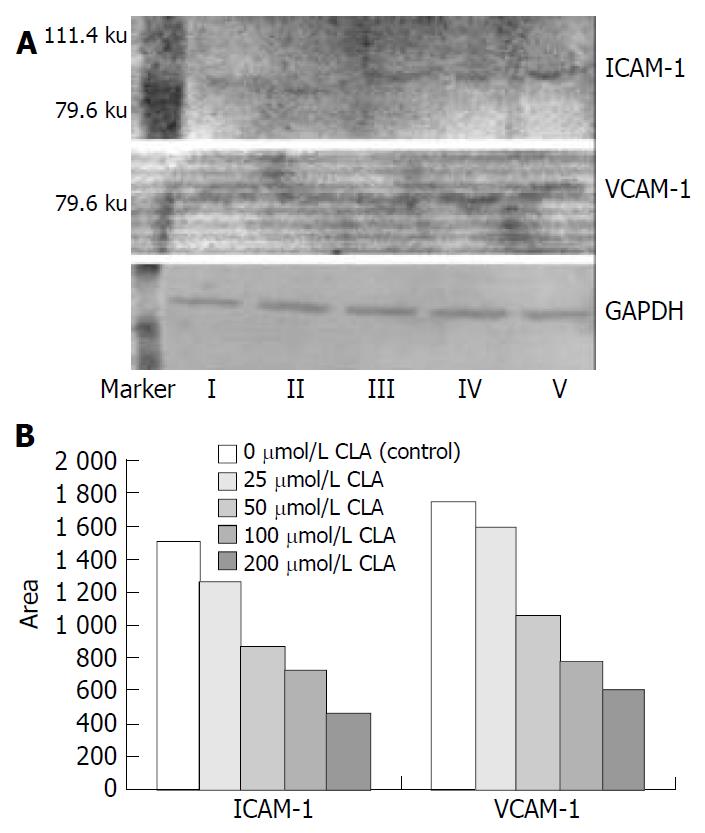
As shown in Figure 2, the expression of ICAM-1 and VCAM-1 protein in SGC-7901 cells treated with different concentrations of c9,t11-CLA was decreased in comparison with that in the negative control group. Analyzed by ChemiImager 4000 digital system, the level of ICAM-1 and VCAM-1 protein in SGC-7901 cells treated with 200 μmol/L c9,t11-CLA increased 70.2% and 65.4% respectively, compared with that in the negative control group.
Many researches have indicated the importance of cancer cell-extracellular matrix (ECM) interaction in tumor metastasis. Cell and matrix interactions could promote cell migration, proliferation, and ECM degradation[47-51]. It also has been shown that prevention of tumor cell adhesion and migration is related to inhibition of tumor cell invasion into the basement membrane. Laminin (LN), fibronectin (FN) and type IV collagen are the principal components of ECM. The in vitro assays of FN, LN, or Matrigel that mainly contain FN, LN and type IV collagen can better simulate the in vivo adhesive process. It was shown that agents that inhibited cell attachment in vitro decreased the invasion and metastatic potential of tumor cells in vivo. Therefore, cell adhesion assay not only is employed in determining the adhesive interaction between tumor cells and matrix components, but also is suitable in screening agents that can inhibit adhesion and metastasis of tumor cell. We demonstrated in our current study that after incubation with 200, 100, and 50 μmol/L of c9,t11-CLA for 1 h, the attachment to extracellular matrix components of SGC-7901 cells was significantly reduced. The result was consistent with the findings in our previous study[44]. Therefore, we could conclude that c9,t11-CLA could inhibit the attachment to extracellular matrix components of tumor cells and this process might be a mechanism for the inhibition of tumor invasion.
Before they can invade or metastasize, tumor cells have to dissociate from primary neoplasms. A loss of cell-cell adhesive interaction is required for the detachment. Thus, adhesion molecules play an important role in metastatic process. Cadherins have been found to be a class of calcium dependent cell adhesion molecules involved in homotypic cell-cell adhesion[7,8]. E-cadherin is a member of the cadherin family that is expressed in all epithelial cells and is essential to the maintenance of cell morphology, cell movement and cell adhesive function. Because E-cadherin can maintain cell adhesion, its abnormalities may be associated with tumorigenesis. It was proved that abnormalities of E-cadherin mRNA and E-cadherin protein expression existed in various human primary cancers, such as gastric, colon, pancreas, esophagus, liver, prostate, bladder, breast, and head and neck tumors[52]. Recent studies showed that gene mutations or loss of heterozygous E-cadherin occurred in gastric carcinomas, ovary cancer, and cervix cancer. It was found that E-cadherin had strong expression in well-differentiated noninvasive cancers with tight cell-cell adhesion, and had markedly reduced, heterogeneous, or even no expression in undifferentiated invasive cancers with lack of cell-cell adhesion. Several studies have offered the evidence that reduction or structural alternation of E-cadherin expression plays a causal role in metastasis of gastric and colon cancers. The role of E-cadherin in metastasis and invasion was further demonstrated by the fact that the invasion of epithelial tumor cell lines was inhibited in vitro by transfection and expression of E-cadherin cDNA, and induced again by exposure to anti-E-cadherin monoclonal antibodies[53]. Through its cytoplasmic sequence, E-cadherin was associated with a group of proteins called catenins, which are necessary for E-cadherin function[54]. Dysfunction of catenin can cause instability of homotypic cell-cell adhesion mediated by E-cadherin. Therefore, in tumors with normal E-cadherin expression, alteration of cell adhesion may result from abnormal expression of catenins. Catenins have been classified into α-catenin, β-catenin, and γ-catenin. α-catenin links E-cadherin with cytoskeleton. Expression of α-catenin is essential to the function of E-cadherin in normal cells. Thus, in cancers with normal E-cadherin expression, decreased expression of α-catenin leads to impaired cell adhesion. Downregulation of α-catenin and E-cadherin expression in several cancer tissues has been found to be associated with differentiation degree, invasion and metastasis of cancer cells[55-57]. Our current study demonstrated that after incubation with different concentrations of c9,t11-CLA for 24 h, expression of E-cadherin and catenin in SGC-7901 cells increased. Through upregulation of expression of E-cadherin and catenin, c9,t11-CLA also increased homotypic adhesion of cancer cells.
ICAM-1 is a 70-110 ku glycoprotein belonging to the immunoglobulin superfamily and is also a ligand for leukocyte-function associated antigen-1 (LFA-1). It has been reported that ICAM-1 could express on the surface of tumor cells, endothelial cells, keratinocytes, and mediate heterotypic cell-cell interaction[58]. Several studies indicated that high expression of ICAM-1 in melanoma cells was associated with tumor metastasis, and expression on the surface of metastasizing cancer cells in lymph node increased significantly[59]. It is suggested that high expression of ICAM-1 on the surface of metastasizing cancer cells in lymph node plays a role in evading immune destruction, thus helping these cancer cells retain in lymphatic sinus to form metastasis. VCAM-1 is a 90 ku cell surface glycoprotein belonging to the immunoglobulin superfamily. Studies also showed that VCAM-1 in renal cell carcinoma, melanoma, and malignant sarcoma linked tumor cells to endothelial cells via binding to integrin α4β1, and contributed to penetration into blood vessels[60]. We demonstrated here that different concentrations of c9,t11-CLA could decrease expression of ICAM-1 and VCAM-1 in SGC-7901 cells after incubation for 24 h and also decrease heterotypic adhesion of cancer cells via downregulation of expression of ICAM-1 and VCAM-1.
In conclusion, c9,t11-CLA can inhibit cell-matrix component interactions, increase expression of E-cadherin and catenin and reduce expression of ICAM-1 and VCAM-1 in SGC-7901 cells. Through these effects, c9,t11-CLA may inhibit the invasion of SGC-7901 cells.
Edited by Chou LF and Wang XL Proofread by Xu FM
| 1. | Liu LX, Liu ZH, Jiang HC, Qu X, Zhang WH, Wu LF, Zhu AL, Wang XQ, Wu M. Profiling of differentially expressed genes in human gastric carcinoma by cDNA expression array. World J Gastroenterol. 2002;8:580-585. [PubMed] |
| 2. | Song ZJ, Gong P, Wu YE. Relationship between the expression of iNOS,VEGF,tumor angiogenesis and gastric cancer. World J Gastroenterol. 2002;8:591-595. [PubMed] |
| 3. | Shi XY, Zhao FZ, Dai X, Ma LS, Dong XY, Fang J. Effect of jianpiyiwei capsule on gastric precancerous lesions in rats. World J Gastroenterol. 2002;8:608-612. [PubMed] |
| 4. | Zhao AG, Zhao HL, Jin XJ, Yang JK, Tang LD. Effects of Chinese Jianpi herbs on cell apoptosis and related gene expression in human gastric cancer grafted onto nude mice. World J Gastroenterol. 2002;8:792-796. [PubMed] |
| 5. | Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 442] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 6. | Hansson LE, Sparén P, Nyrén O. Survival in stomach cancer is improving: results of a nationwide population-based Swedish study. Ann Surg. 1999;230:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2396] [Cited by in RCA: 2422] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 8. | Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 627] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 9. | Sébédio JL, Gnaedig S, Chardigny JM. Recent advances in conjugated linoleic acid research. Curr Opin Clin Nutr Metab Care. 1999;2:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Pariza MW, Park Y, Cook ME. Mechanisms of action of conjugated linoleic acid: evidence and speculation. Proc Soc Exp Biol Med. 2000;223:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 214] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Pariza MW, Park Y, Cook ME. Conjugated linoleic acid and the control of cancer and obesity. Toxicol Sci. 1999;52:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Whigham LD, Cook ME, Atkinson RL. Conjugated linoleic acid: implications for human health. Pharmacol Res. 2000;42:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 163] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | MacDonald HB. Conjugated linoleic acid and disease prevention: a review of current knowledge. J Am Coll Nutr. 2000;19:111S-118S. [PubMed] |
| 14. | Banni S. Conjugated linoleic acid metabolism. Curr Opin Lipidol. 2002;13:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Belury MA. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Annu Rev Nutr. 2002;22:505-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 515] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 16. | Belury MA. Inhibition of carcinogenesis by conjugated linoleic acid: potential mechanisms of action. J Nutr. 2002;132:2995-2998. [PubMed] |
| 17. | Pariza MW, Ashoor SH, Chu FS, Lund DB. Effects of temperature and time on mutagen formation in pan-fried hamburger. Cancer Lett. 1979;7:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 130] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Pariza MW, Hargraves WA. A beef-derived mutagenesis modulator inhibits initiation of mouse epidermal tumors by 7,12-dimethylbenz[a]anthracene. Carcinogenesis. 1985;6:591-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 150] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Ha YL, Grimm NK, Pariza MW. Anticarcinogens from fried ground beef: heat-altered derivatives of linoleic acid. Carcinogenesis. 1987;8:1881-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 554] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 20. | Belury MA, Nickel KP, Bird CE, Wu Y. Dietary conjugated linoleic acid modulation of phorbol ester skin tumor promotion. Nutr Cancer. 1996;26:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 134] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Ha YL, Storkson J, Pariza MW. Inhibition of benzo(a)pyrene-induced mouse forestomach neoplasia by conjugated dienoic derivatives of linoleic acid. Cancer Res. 1990;50:1097-1101. [PubMed] |
| 22. | Zhu Y, Qiou J, Chen B. [The inhibitory effect of CLA on mice forestomach neoplasia induced by B(a)P]. Zhonghua Yufang Yixue Zazhi. 2001;35:19-22. [PubMed] |
| 23. | Chen BQ, Xue YB, Liu JR, Yang YM, Zheng YM, Wang XL, Liu RH. Inhibition of conjugated linoleic acid on mouse forestomach neoplasia induced by benzo (a) pyrene and chemopreventive mechanisms. World J Gastroenterol. 2003;9:44-49. [PubMed] |
| 24. | Ip C, Jiang C, Thompson HJ, Scimeca JA. Retention of conjugated linoleic acid in the mammary gland is associated with tumor inhibition during the post-initiation phase of carcinogenesis. Carcinogenesis. 1997;18:755-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Ip C, Singh M, Thompson HJ, Scimeca JA. Conjugated linoleic acid suppresses mammary carcinogenesis and proliferative activity of the mammary gland in the rat. Cancer Res. 1994;54:1212-1215. [PubMed] |
| 26. | Ip C, Banni S, Angioni E, Carta G, McGinley J, Thompson HJ, Barbano D, Bauman D. Conjugated linoleic acid-enriched butter fat alters mammary gland morphogenesis and reduces cancer risk in rats. J Nutr. 1999;129:2135-2142. [PubMed] |
| 27. | Thompson H, Zhu Z, Banni S, Darcy K, Loftus T, Ip C. Morphological and biochemical status of the mammary gland as influenced by conjugated linoleic acid: implication for a reduction in mammary cancer risk. Cancer Res. 1997;57:5067-5072. [PubMed] |
| 28. | Banni S, Angioni E, Casu V, Melis MP, Carta G, Corongiu FP, Thompson H, Ip C. Decrease in linoleic acid metabolites as a potential mechanism in cancer risk reduction by conjugated linoleic acid. Carcinogenesis. 1999;20:1019-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 126] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Kimoto N, Hirose M, Futakuchi M, Iwata T, Kasai M, Shirai T. Site-dependent modulating effects of conjugated fatty acids from safflower oil in a rat two-stage carcinogenesis model in female Sprague-Dawley rats. Cancer Lett. 2001;168:15-21. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Ip C, Ip MM, Loftus T, Shoemaker S, Shea-Eaton W. Induction of apoptosis by conjugated linoleic acid in cultured mammary tumor cells and premalignant lesions of the rat mammary gland. Cancer Epidemiol Biomarkers Prev. 2000;9:689-696. [PubMed] |
| 31. | Liew C, Schut HA, Chin SF, Pariza MW, Dashwood RH. Protection of conjugated linoleic acids against 2-amino-3-methylimidazo[4,5-f]quinoline-induced colon carcinogenesis in the F344 rat: a study of inhibitory mechanisms. Carcinogenesis. 1995;16:3037-3043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 191] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Visonneau S, Cesano A, Tepper SA, Scimeca JA, Santoli D, Kritchevsky D. Conjugated linoleic acid suppresses the growth of human breast adenocarcinoma cells in SCID mice. Anticancer Res. 1997;17:969-973. [PubMed] |
| 33. | Cesano A, Visonneau S, Scimeca JA, Kritchevsky D, Santoli D. Opposite effects of linoleic acid and conjugated linoleic acid on human prostatic cancer in SCID mice. Anticancer Res. 1998;18:1429-1434. [PubMed] |
| 34. | Shultz TD, Chew BP, Seaman WR, Luedecke LO. Inhibitory effect of conjugated dienoic derivatives of linoleic acid and beta-carotene on the in vitro growth of human cancer cells. Cancer Lett. 1992;63:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 151] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Igarashi M, Miyazawa T. Newly recognized cytotoxic effect of conjugated trienoic fatty acids on cultured human tumor cells. Cancer Lett. 2000;148:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Igarashi M, Miyazawa T. The growth inhibitory effect of conju-gated linoleic acid on a human hepatoma cell line, HepG2, is induced by a change in fatty acid metabolism, but not the facili-tation of lipid peroxidation in the cells. Biochim Biophys Acta. 2001;1530:162-171. [RCA] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Park Y, Allen KG, Shultz TD. Modulation of MCF-7 breast cancer cell signal transduction by linoleic acid and conjugated linoleic acid in culture. Anticancer Res. 2000;20:669-676. [PubMed] |
| 38. | O'Shea M, Devery R, Lawless F, Murphy J, Stanton C. Milk fat conjugated linoleic acid (CLA) inhibits growth of human mammary MCF-7 cancer cells. Anticancer Res. 2000;20:3591-3601. [PubMed] |
| 39. | O'Shea M, Stanton C, Devery R. Antioxidant enzyme defence responses of human MCF-7 and SW480 cancer cells to conjugated linoleic acid. Anticancer Res. 1999;19:1953-1959. [PubMed] |
| 40. | Cunningham DC, Harrison LY, Shultz TD. Proliferative responses of normal human mammary and MCF-7 breast cancer cells to linoleic acid, conjugated linoleic acid and eicosanoid synthesis inhibitors in culture. Anticancer Res. 1997;17:197-203. [PubMed] |
| 41. | Schønberg S, Krokan HE. The inhibitory effect of conjugated dienoic derivatives (CLA) of linoleic acid on the growth of human tumor cell lines is in part due to increased lipid peroxidation. Anticancer Res. 1995;15:1241-1246. [PubMed] |
| 42. | Liu J, Chen B, Liu R, Lu G. [Inhibitory effect of conjugated linoleic acid on human gastric carcinoma cell line]. Weisheng Yanjiu. 1999;28:353-355. [PubMed] |
| 43. | Liu JR, Li BX, Chen BQ, Han XH, Xue YB, Yang YM, Zheng YM, Liu RH. Effect of cis-9, trans-11-conjugated linoleic acid on cell cycle of gastric adenocarcinoma cell line (SGC-7901). World J Gastroenterol. 2002;8:224-229. [PubMed] |
| 44. | Xue Y, Chen B, Zheng Y, Yuan L. [Effects of conjugated linoleic acid on the metastasis of mouse melanoma B16-MB]. Weisheng Yanjiu. 2001;30:37-39. [PubMed] |
| 45. | Yang Y, Chen B, Xue Y, Zheng Y. [Effects of c9, t11-conjugated linoleic acid on the metastasis of human gastric carcinoma cell line]. Weisheng Yanjiu. 2003;32:117-119. [PubMed] |
| 46. | Yang YM, Chen BQ, Zheng YM, Wang XL, Liu JR, Xue YB, Liu RH. [The effects of conjugated linoleic acid on the expression of invasiveness and metastasis-associated gene of human gastric carcinoma cell line]. Zhonghua Yufang Yixue Zazhi. 2003;37:26-28. [PubMed] |
| 47. | Yoon SO, Kim MM, Chung AS. Inhibitory effect of selenite on invasion of HT1080 tumor cells. J Biol Chem. 2001;276:20085-20092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Ara T, Deyama Y, Yoshimura Y, Higashino F, Shindoh M, Matsumoto A, Fukuda H. Membrane type 1-matrix metalloproteinase expression is regulated by E-cadherin through the suppression of mitogen-activated protein kinase cascade. Cancer Lett. 2000;157:115-121. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Seftor RE, Seftor EA, Gehlsen KR, Stetler-Stevenson WG, Brown PD, Ruoslahti E, Hendrix MJ. Role of the alpha v beta 3 integrin in human melanoma cell invasion. Proc Natl Acad Sci USA. 1992;89:1557-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 310] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 50. | Nakahara H, Nomizu M, Akiyama SK, Yamada Y, Yeh Y, Chen WT. A mechanism for regulation of melanoma invasion. Liga-tion of alpha6beta1 integrin by laminin G peptides. J Biol Chem. 1996;271:27221-27224. [RCA] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3412] [Cited by in RCA: 3286] [Article Influence: 126.4] [Reference Citation Analysis (0)] |
| 52. | Doğan A, Wang ZD, Spencer J. E-cadherin expression in intestinal epithelium. J Clin Pathol. 1995;48:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Chan AO, Lam SK, Chu KM, Lam CM, Kwok E, Leung SY, Yuen ST, Law SY, Hui WM, Lai KC. Soluble E-cadherin is a valid prognostic marker in gastric carcinoma. Gut. 2001;48:808-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Jawhari A, Farthing M, Pignatelli M. The importance of the E-cadherin-catenin complex in the maintenance of intestinal epithelial homoeostasis: more than intercellular glue? Gut. 1997;41:581-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Jiang WG. E-cadherin and its associated protein catenins, cancer invasion and metastasis. Br J Surg. 1996;83:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 90] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 56. | Yu J, Ebert MP, Miehlke S, Rost H, Lendeckel U, Leodolter A, Stolte M, Bayerdorffer E, Malfertheiner P. Alpha-catenin expres-sion is decreased in human gastric cancers and in the gastric mucosa of first degree relatives. Gut. 2000;46:639-644. [RCA] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 57. | Shiozaki H, Iihara K, Oka H, Kadowaki T, Matsui S, Gofuku J, Inoue M, Nagafuchi A, Tsukita S, Mori T. Immunohistochemical detection of alpha-catenin expression in human cancers. Am J Pathol. 1994;144:667-674. [PubMed] |
| 58. | Schwaeble W, Kerlin M, Meyer zum Büschenfelde KH, Dippold W. De novo expression of intercellular adhesion molecule 1 (ICAM-1, CD54) in pancreas cancer. Int J Cancer. 1993;53:328-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 59. | Natali P, Nicotra MR, Cavaliere R, Bigotti A, Romano G, Temponi M, Ferrone S. Differential expression of intercellular adhesion molecule 1 in primary and metastatic melanoma lesions. Cancer Res. 1990;50:1271-1278. [PubMed] |
| 60. | Tomita Y, Saito T, Saito K, Oite T, Shimizu F, Sato S. Possible significance of VLA-4 (alpha 4 beta 1) for hematogenous metastasis of renal-cell cancer. Int J Cancer. 1995;60:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |