Copyright
©The Author(s) 2003.
World J Gastroenterol. Sep 15, 2003; 9(9): 2073-2077
Published online Sep 15, 2003. doi: 10.3748/wjg.v9.i9.2073
Published online Sep 15, 2003. doi: 10.3748/wjg.v9.i9.2073
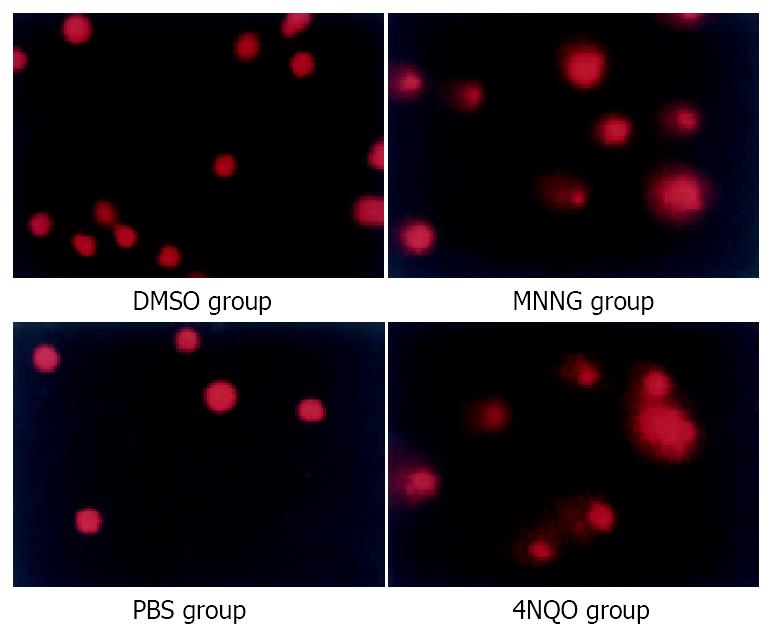
Figure 1 Comet assays indicated accumulation of strand breaks.
With low concentration MNNG 0.2 μM 2.5 h treatment, SCGE showed significant comet tail formation, just like the positive control group (4NQO 2.5 mM 30 min). While the control group, both the DMSO treatment group and PBS treatment group, showed no comet tail formation.
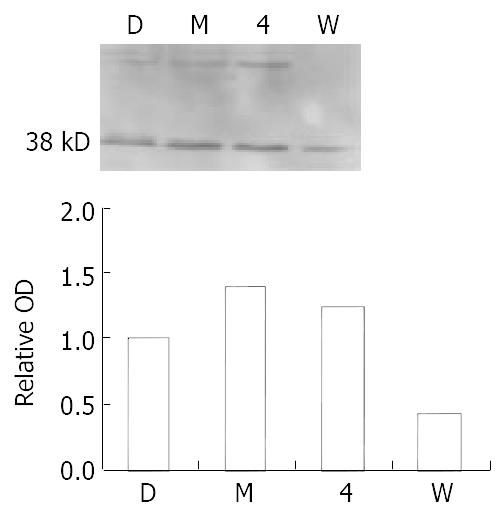
Figure 2 MNNG exposure activates ATM/ATR in Vero cells.
Increased phosphorylation of ATM/ATR was observed after 0.2 μM MNNG 2.5 h exposure. One activated substrate mo-lecular weight is about 38 kDa. Pretreatment with 100 μM wortmannin abrogated the upregulation. Positive control 4-NQO 2.5 mM 30 minutes.
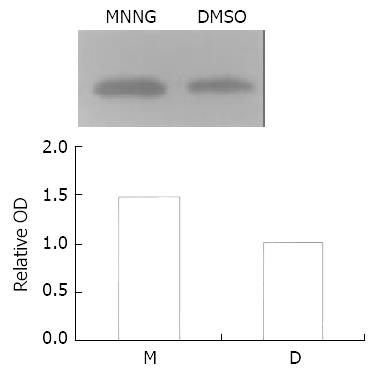
Figure 3 MNNG treatment activated p38 kinase.
p38 kinase in Vero cells was activated by about 1.47 fold after the exposure to 0.2 μmol/L MNNG for 2.5 h.
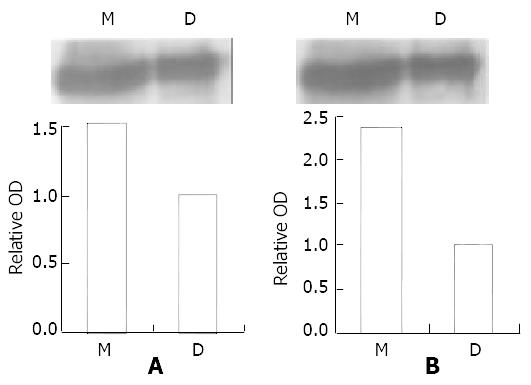
Figure 4 ATM/ATR-P38MAPK pathway is activated by MNNG.
Immunoprecipitation showed phospho-ATM/ATR substrate antibody connected with both the p38 MAPK antibody and the phospho-p38MAPK antibody. The band of MNNG group is 1.78 fold (p38MAPK) and 2.37 fold (phospho-p38MAPK) stron-ger than DMSO group. Combined with the result that P38MAPK was activated by the same treatment, we conclude that ATM/ATR-P38MAPK pathway is activated by MNNG.
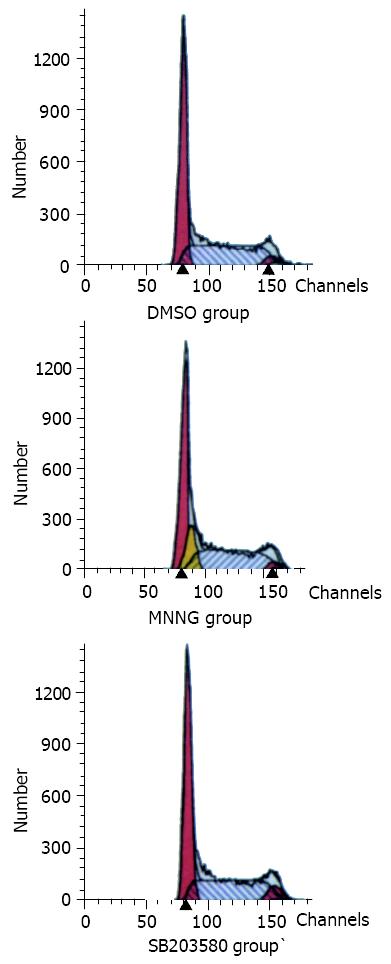
Figure 5 P38MAPK joined G1-S arrest.
In the flow cytometry analysis work, it was found that the population of S phase of cell cycle in MNNG treatment group was decreased as com-pared with the controls (DMSO group). Pretreatment with p38MAPK specific inhibitor SB203580 for 1 h, the G1-S arrest disappeared after the same MNNG treatment.
- Citation: Zhu KQ, Zhang SJ. Involvement of ATM/ATR-p38 MAPK cascade in MNNG induced G1-S arrest. World J Gastroenterol 2003; 9(9): 2073-2077
- URL: https://www.wjgnet.com/1007-9327/full/v9/i9/2073.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i9.2073