Copyright
©The Author(s) 2024.
World J Gastroenterol. Nov 14, 2024; 30(42): 4532-4543
Published online Nov 14, 2024. doi: 10.3748/wjg.v30.i42.4532
Published online Nov 14, 2024. doi: 10.3748/wjg.v30.i42.4532
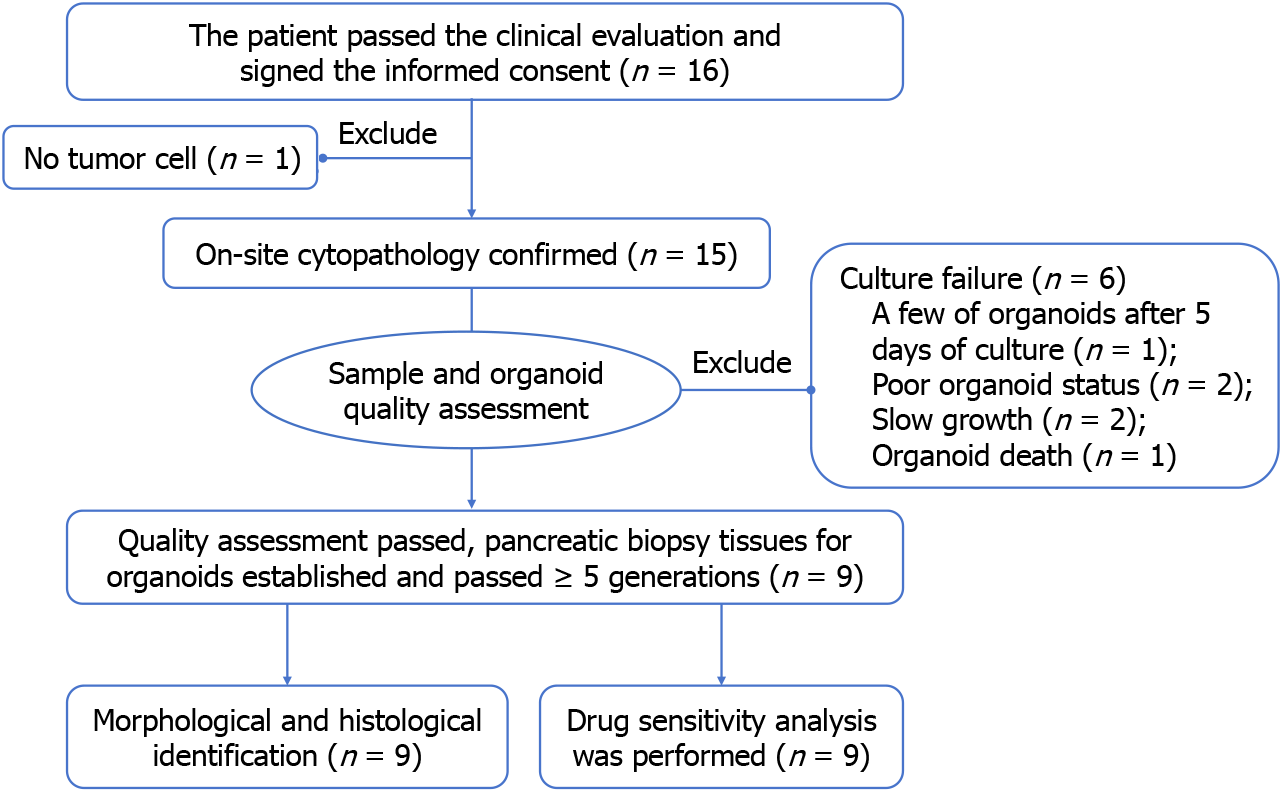
Figure 1 Study scheme.
Finally, 9 patients successfully constructed organoids and completed identification and drug screening.
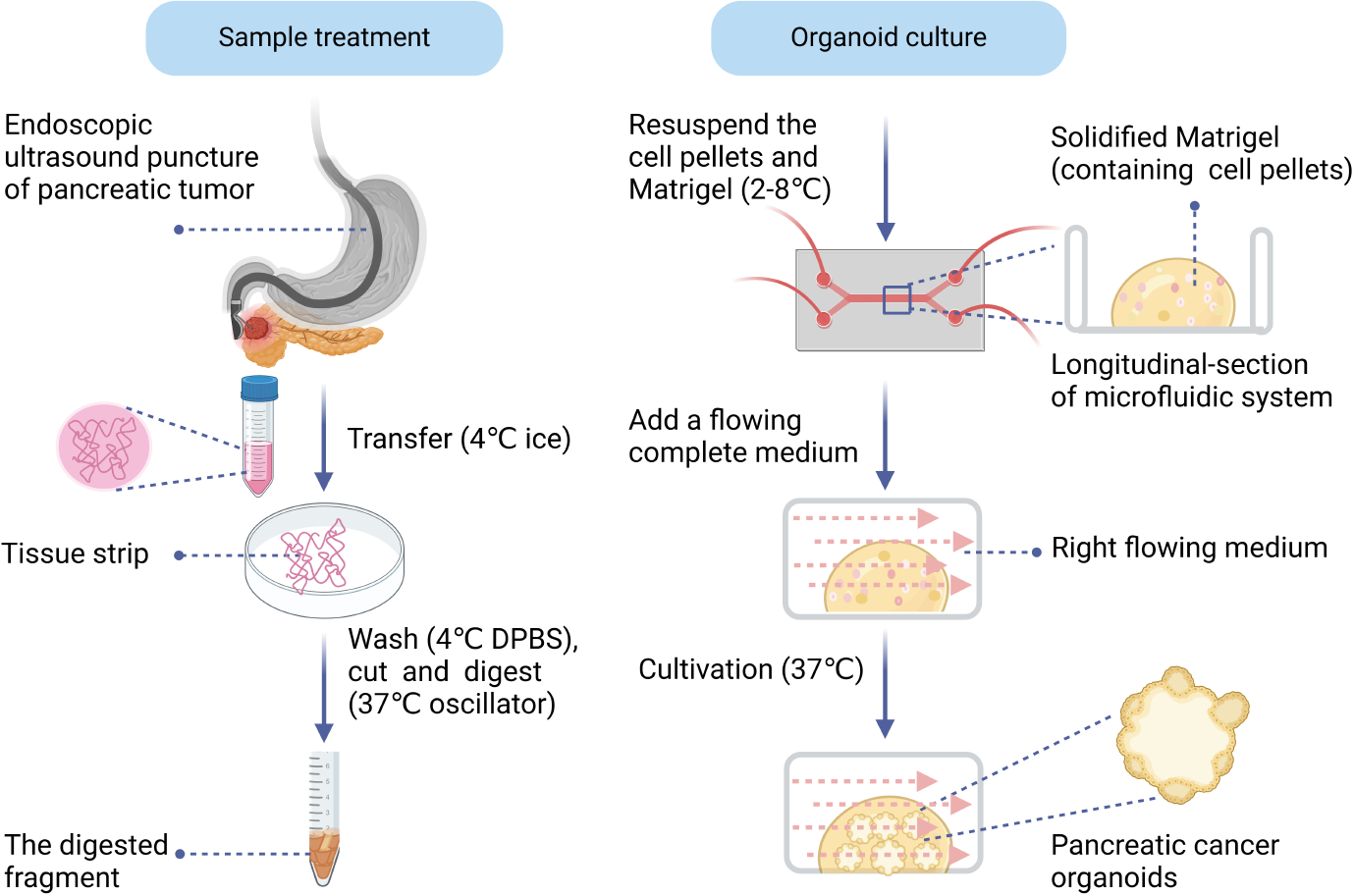
Figure 2 Schematic diagram of sample treatment from endoscopic ultrasound-guided fine-needle biopsy and organoid culture process.
DPBS: Dulbecco’s phosphate-buffered saline.
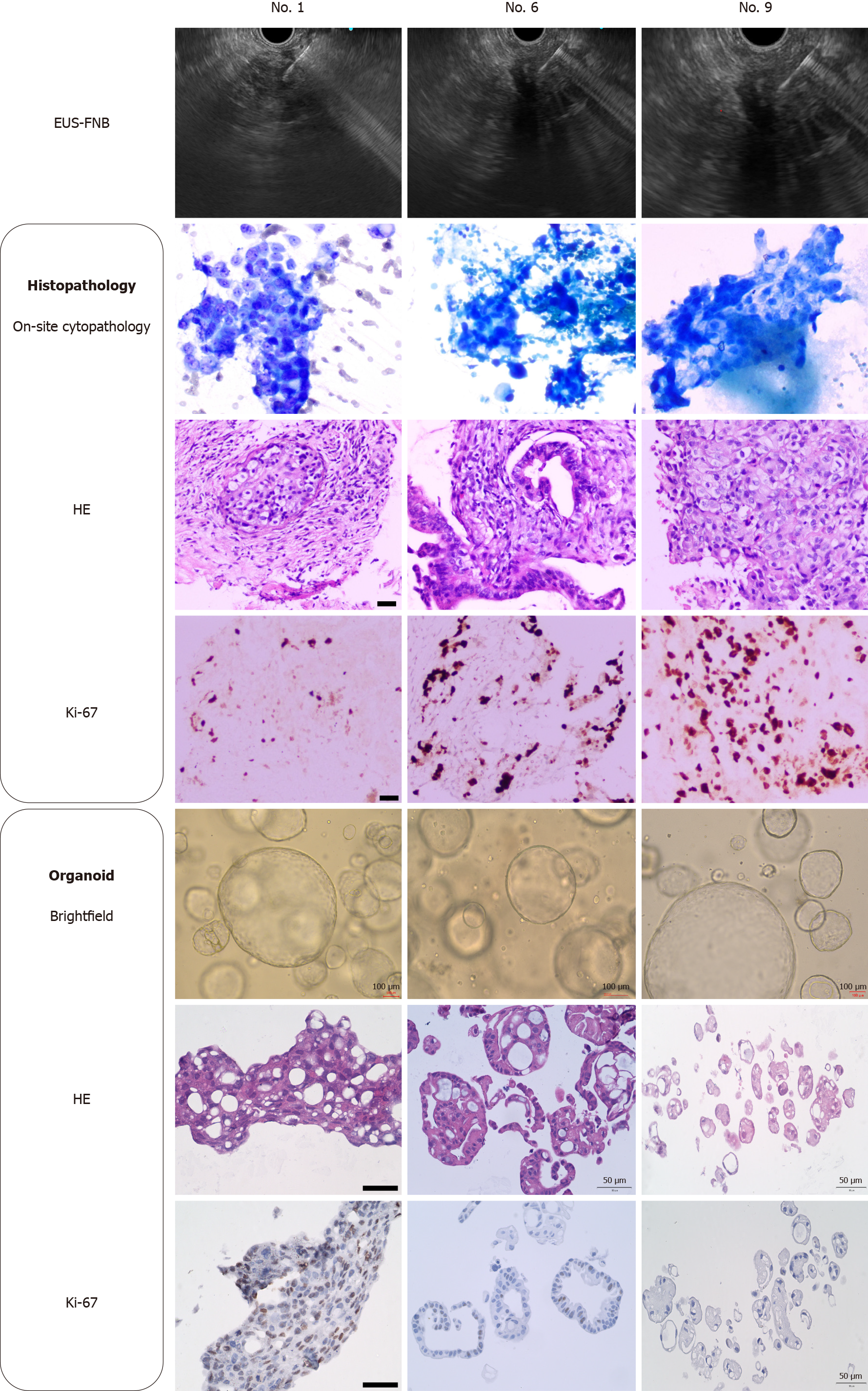
Figure 3 Histological identification of biopsy tissue and organoids from three samples (patient No.
1, No. 6 and No. 9). Bright-field images of pancreatic cancer organoids taken at 4 days of culture. Scale bars of organoid bright-field: 100 µm, scale bars of hematoxylin-eosin staining and Ki-67: 50 µm. EUS-FNB: Endoscopic ultrasound-guided fine-needle biopsy; HE: Hematoxylin-eosin staining.
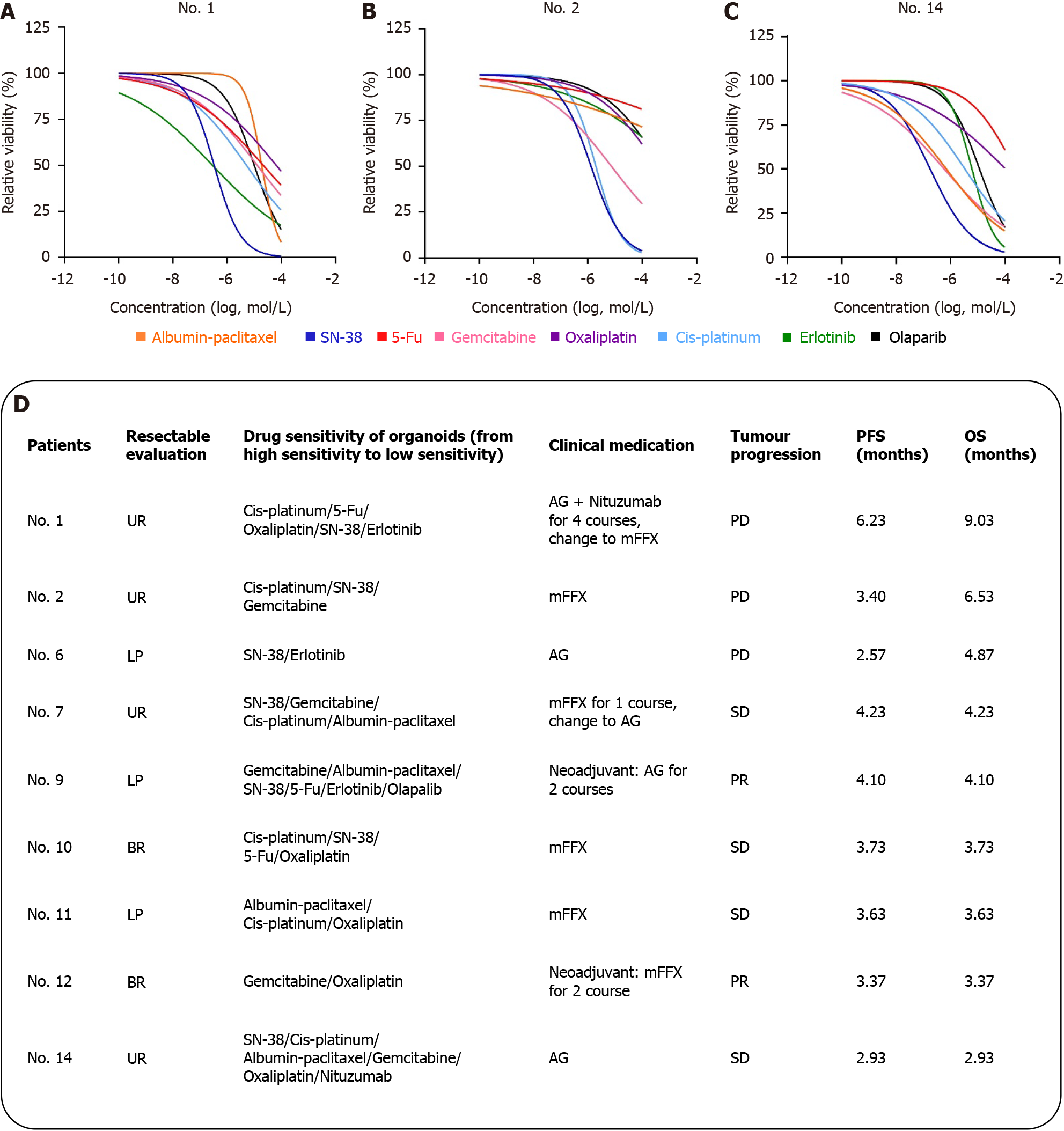
Figure 4 Drug sensitivity analysis and clinical relevance of organoids.
A: Curve drug sensitivity curves of patients No. 1; B: Curve drug sensitivity curves of patients No. 2; C: Curve drug sensitivity curves of patients No. 14; D: Listed the sensitive drugs and clinical protocols for No. 9 patients. Assessment of resectable patients according to National Comprehensive Cancer Network guidelines: Unresectable, local progression, borderline resectable. Patient tumor response was measured using RESIST 1.1 criteria and categorized as partial response, stable disease, and progressive disease. AG: Gemcitabine combined with albumin-paclitaxel; mFFX: Modified FOLFIRINOX, oxaliplatin, irinotecan, fluorouracil and calcium leucovorin; UR: Unresectable; LP: Local progression; BR: Borderline resectable; 5-Fu: Fluorouracil; PD: Progressive disease; SD: Stable disease; PR: Partial response; OS: Overall survival; PFS: Progression-free survival.
- Citation: Yang JL, Zhang JF, Gu JY, Gao M, Zheng MY, Guo SX, Zhang T. Strategic insights into the cultivation of pancreatic cancer organoids from endoscopic ultrasonography-guided biopsy tissue. World J Gastroenterol 2024; 30(42): 4532-4543
- URL: https://www.wjgnet.com/1007-9327/full/v30/i42/4532.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i42.4532