Copyright
©The Author(s) 2024.
World J Gastroenterol. Sep 21, 2024; 30(35): 3942-3953
Published online Sep 21, 2024. doi: 10.3748/wjg.v30.i35.3942
Published online Sep 21, 2024. doi: 10.3748/wjg.v30.i35.3942
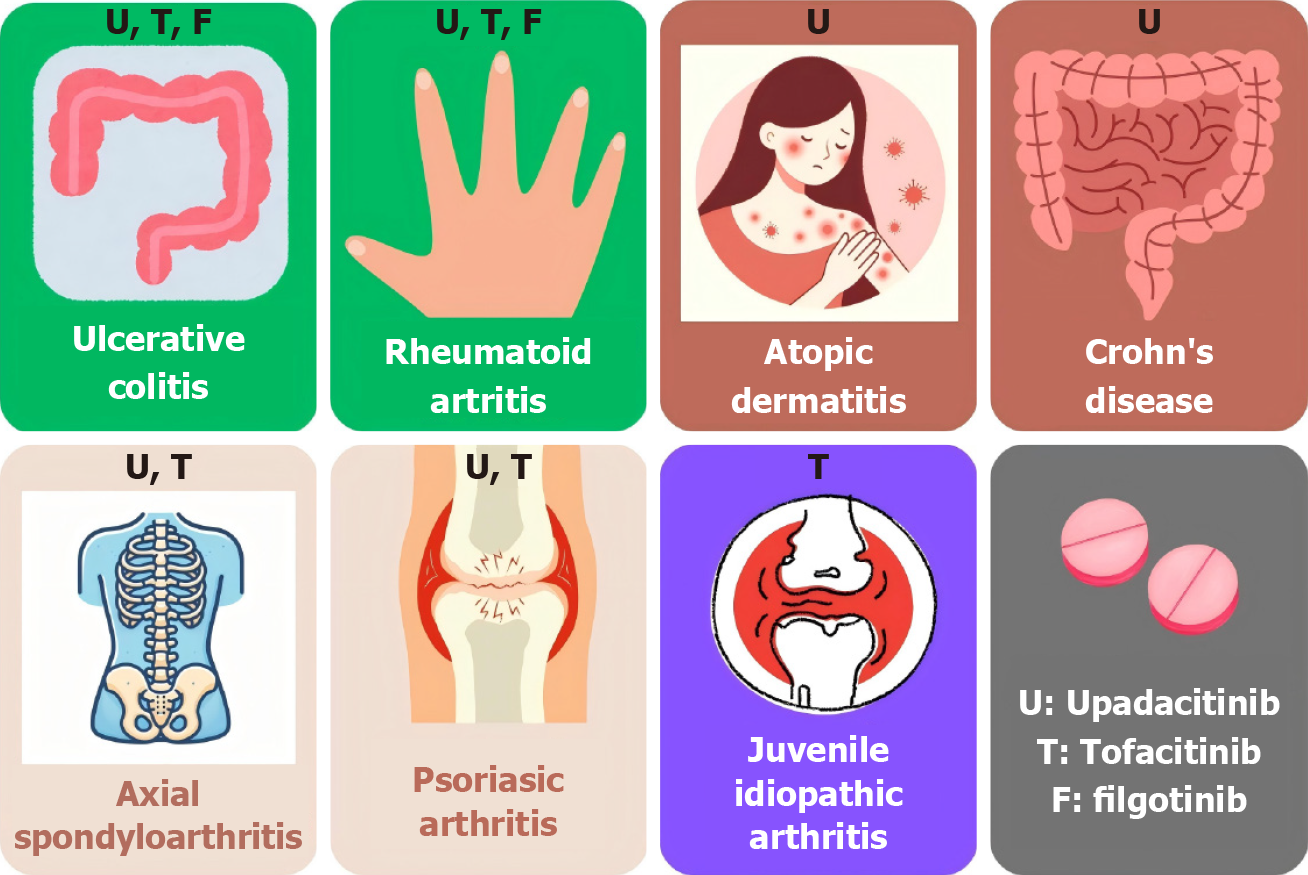
Figure 1 Janus kinase approved for ulcerative colitis and other immunomediated diseases by the United States Food and Drug Administration, European Medicines Agency, and Pharmaceuticals and Medical Devices Agency, except filgotinib, which is only approved by the European Medicines Agency and Pharmaceuticals and Medical Devices Agency.
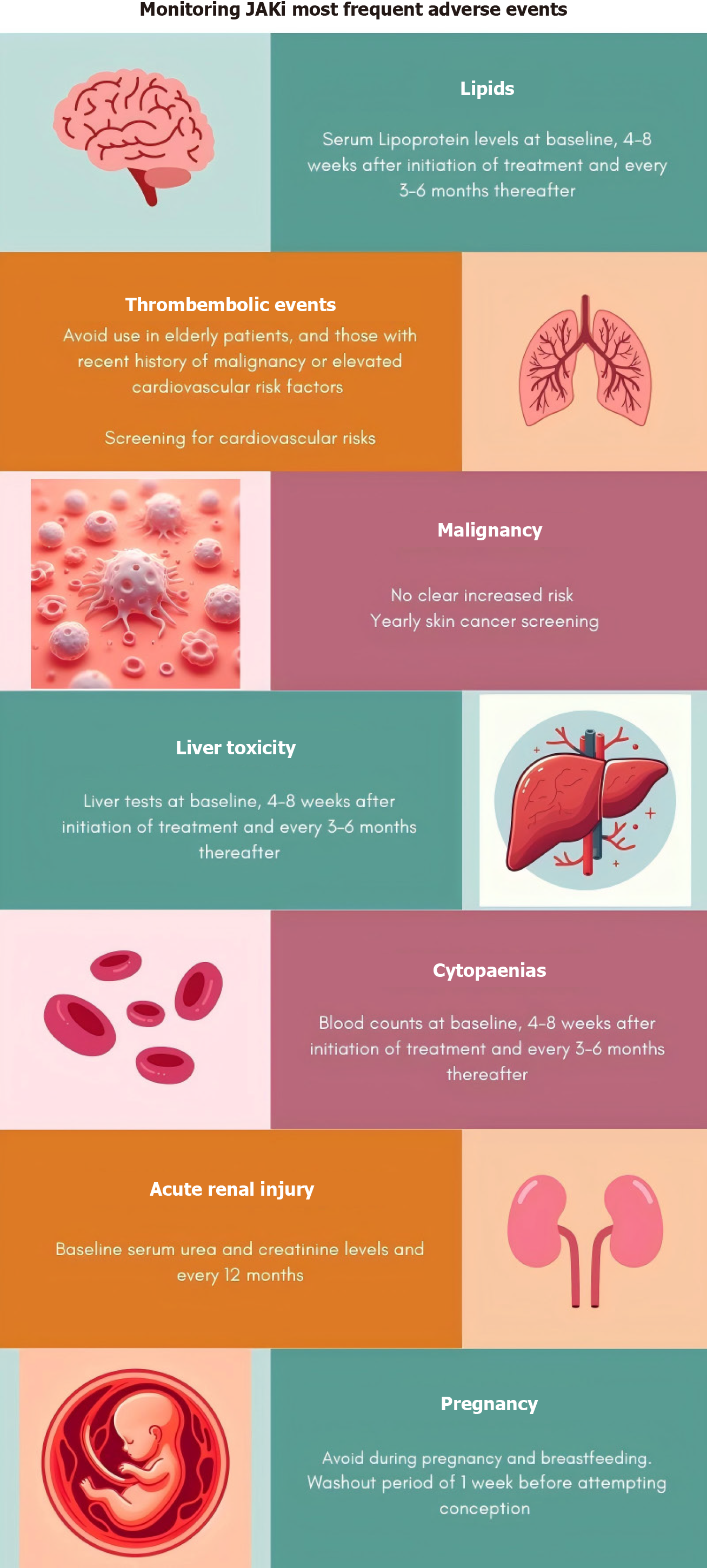
Figure 2 Most frequent adverse events related to Janus kinase inhibitors and how to manage them.
- Citation: Caballero-Mateos AM, Cañadas-de la Fuente GA. Game changer: How Janus kinase inhibitors are reshaping the landscape of ulcerative colitis management. World J Gastroenterol 2024; 30(35): 3942-3953
- URL: https://www.wjgnet.com/1007-9327/full/v30/i35/3942.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i35.3942