Copyright
©The Author(s) 2024.
World J Gastroenterol. Jul 28, 2024; 30(28): 3428-3446
Published online Jul 28, 2024. doi: 10.3748/wjg.v30.i28.3428
Published online Jul 28, 2024. doi: 10.3748/wjg.v30.i28.3428
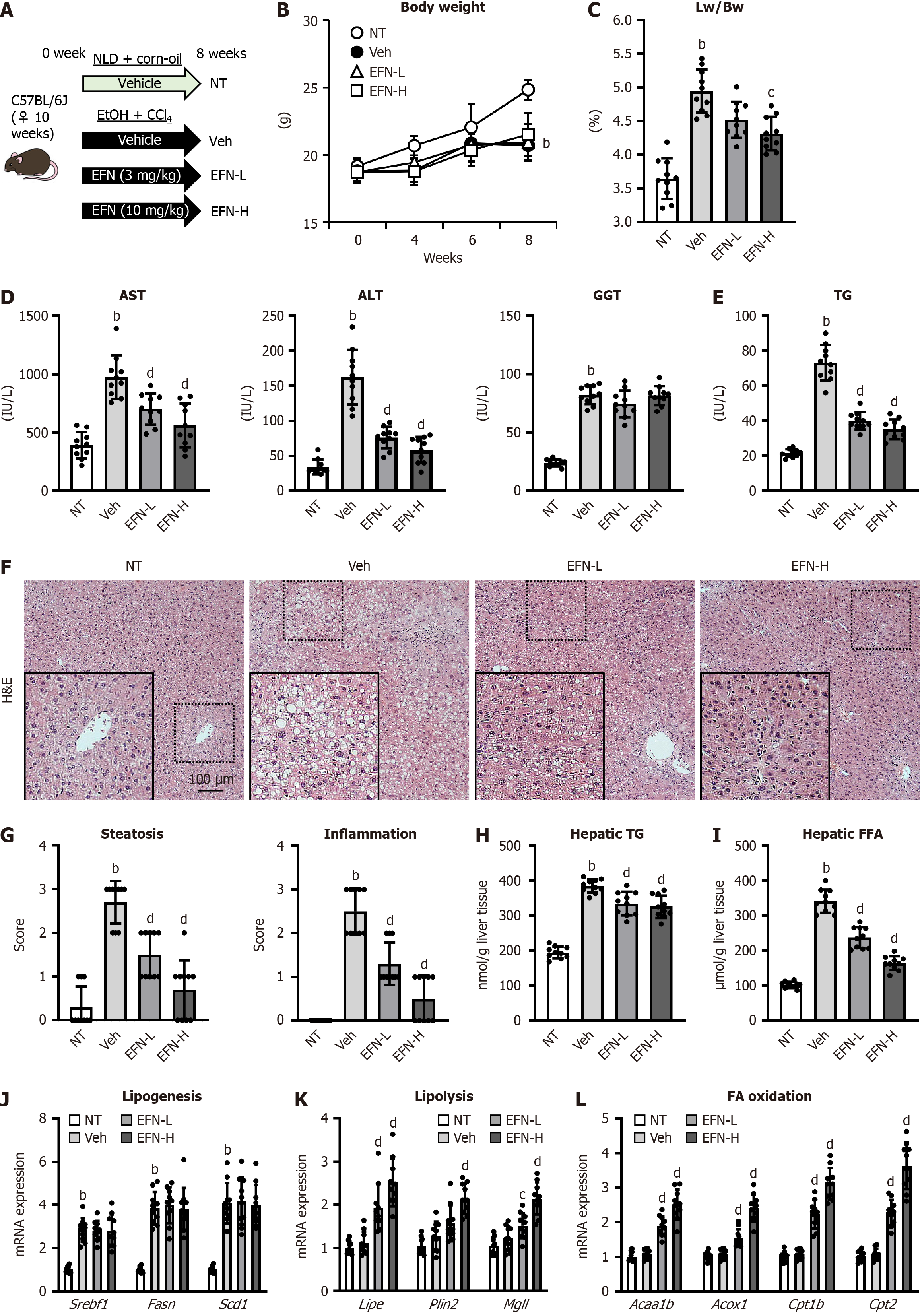
Figure 1 Elafibranor on steatohepatitis and lipid accumulation in the alcohol-associated liver disease mice.
A: In vivo experimental design; B: Changes in the body weights during the experimental period (n = 10); C: Liver/body weight at the end of experiment (n = 10); D: Serum levels of aspartate aminotransferase, alanine aminotransferase and gamma glutamyl transferase (n = 10); E: Serum triglyceride level (n = 10); F: Representative microphotographs of hematoxylin and eosin of the livers in the experimental mice; G: Hepatic pathological scores for steatosis and inflammation. Localized magnified images in the lower left corner of each picture (n = 10); H: Hepatic triglyceride content; I: Hepatic free fatty acid concentration (n = 10); J-L: Hepatic mRNA level of the markers related to lipogenesis (J), lipolysis (K), fatty acid oxidation (L) (n = 10). Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control for real-time quantitative polymerase chain reaction. Quantitative values are indicated as fold changes to the values of non-therapeutic group. Data are the mean ± SD. bP < 0.01 vs non-therapeutic group; cP < 0.05 vs vehicle-treated alcohol-associated liver disease group; dP < 0.01 vs vehicle-treated alcohol-associated liver disease group, significant difference between groups by Student’s t-test. NT: Non-therapeutic group; Veh: Vehicle-treated alcohol-associated liver disease group; EFN-L: Elafiblanor (3 mg/kg/day)-treated alcohol-associated liver disease group; EFN-H: Elafibranor (10 mg/kg/day)-treated alcohol-associated liver disease group; NLD: Normal liquid diet; EtOH: Ethanol; CCL4: Carbon tetrachloride; EFN: Elafiblanor; Lw: Liver weight; Bw: Body weight; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; GGT: Gamma glutamyl transferase; TG: Triglyceride; H&E: Hematoxylin and eosin; FFA: Free fatty acid; FA: Fatty acid.
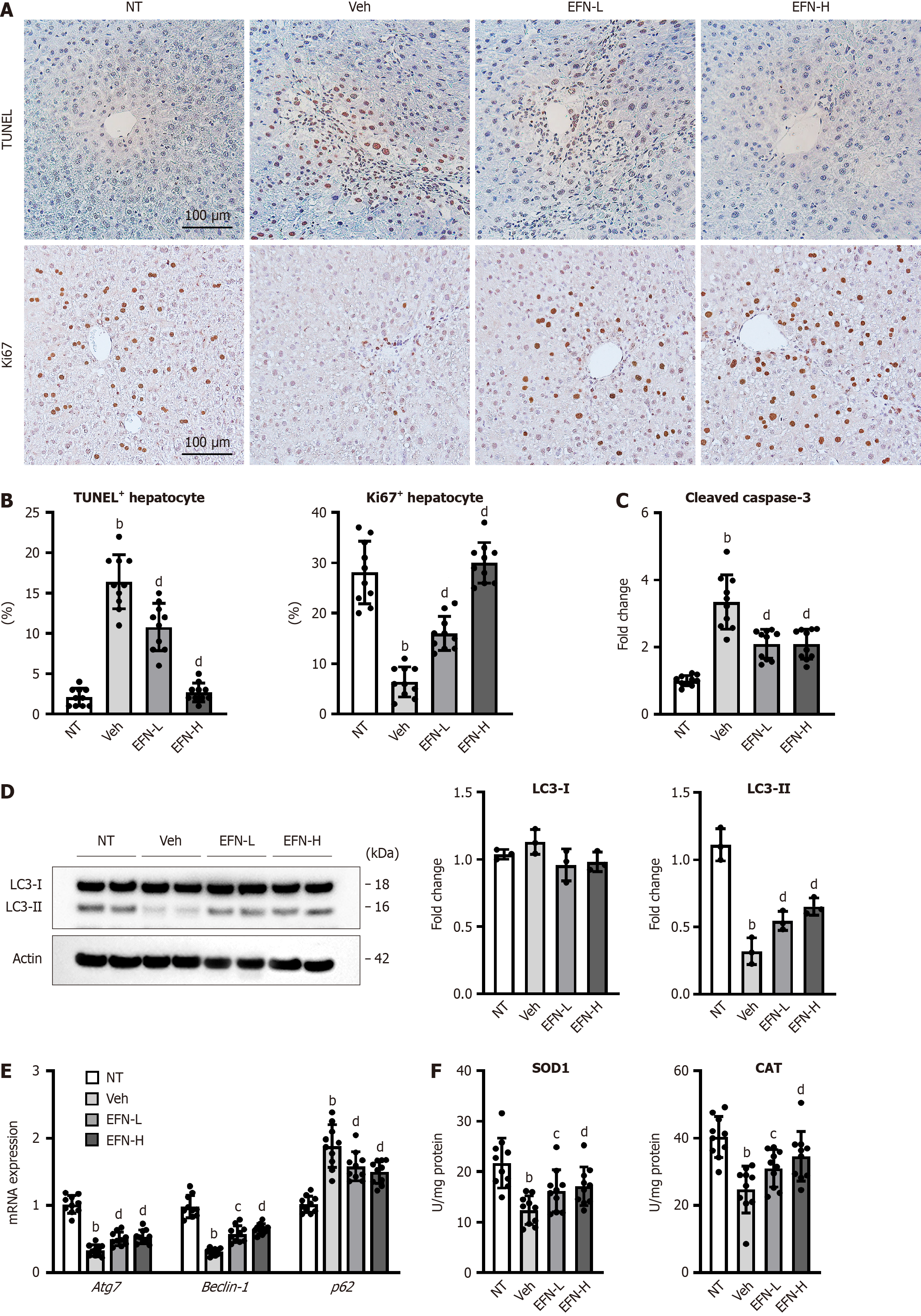
Figure 2 Elafibranor on hepatocyte cell death, autophagy and oxidative stress in the alcohol-associated liver disease mice.
A: Representative microphotographs of TdT-mediated dUTP Nick End Labeling (TUNEL) and Ki67 staining of the livers in the experimental mice; B: Quantification of TUNEL-positive hepatocytes and Ki67-positive hepatocytes in high-power field (n = 10); C: Cleaved caspase-3 level in the liver tissue (n = 10); D: Western blot for LC3-1 and 2 protein level in the liver tissue. Actin was used as an internal control (n = 3); E: Hepatic mRNA level of the markers related to autophagy (n = 10); F: Hepatic level of antioxidant enzymes, superoxide dismutase 1 and catalase (n = 10). Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control for real-time quantitative polymerase chain reaction (E and F). Quantitative values are indicated as fold changes to the values of non-therapeutic group (C-E). Data are the mean ± SD. bP < 0.01 vs non-therapeutic group; cP < 0.05 vs vehicle-treated alcohol-associated liver disease group; dP < 0.01 vs vehicle-treated alcohol-associated liver disease group, significant difference between groups by Student’s t-test. NT: Non-therapeutic group; Veh: Vehicle-treated alcohol-associated liver disease group; EFN-L: Elafiblanor (3 mg/kg/day)-treated alcohol-associated liver disease group; EFN-H: Elafibranor (10 mg/kg/day)-treated alcohol-associated liver disease group; TUNEL: TdT-mediated dUTP Nick End Labeling; SOD: Superoxide dismutase; CAT: Catalase.
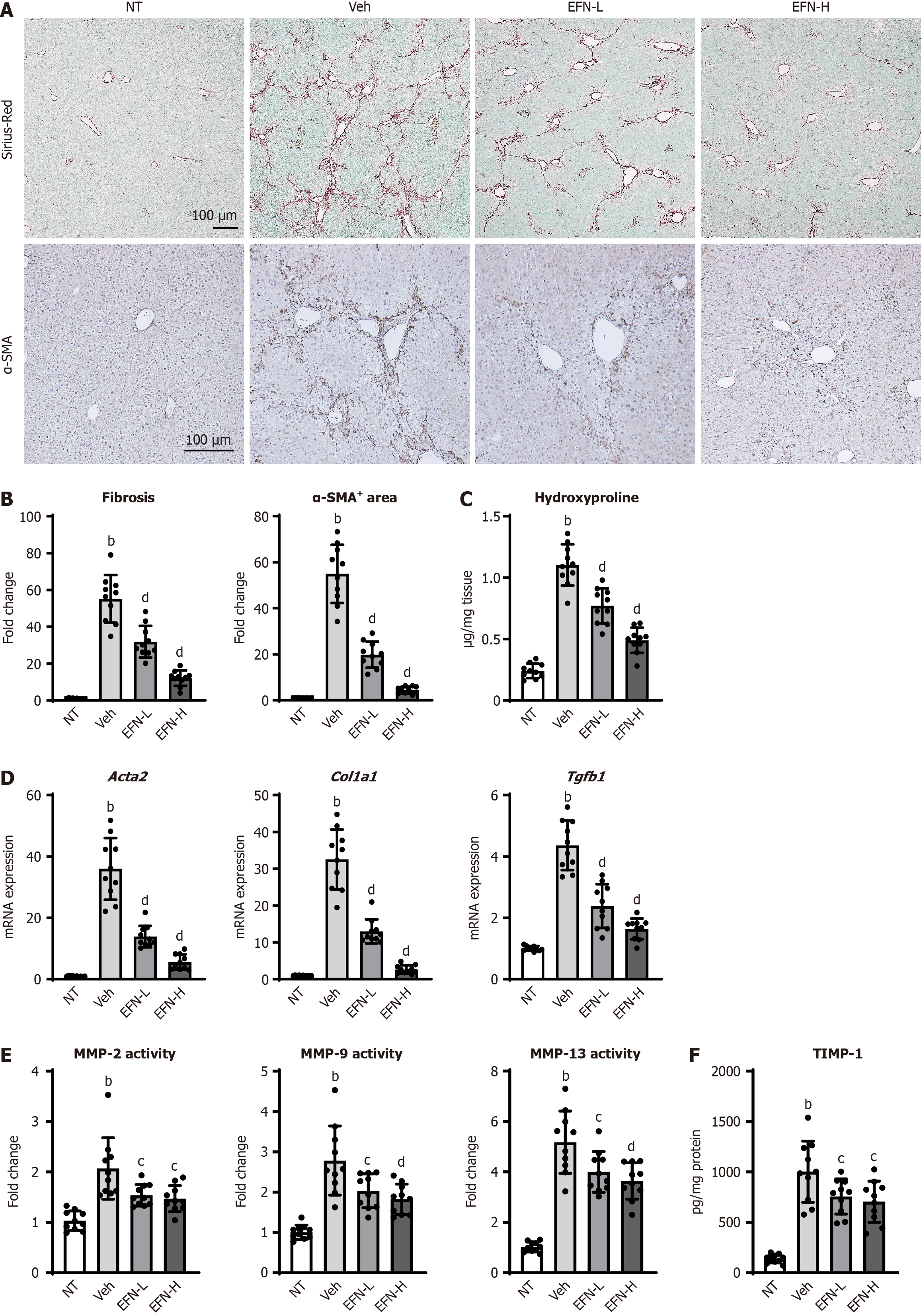
Figure 3 Elafibranor on hepatic fibrosis development in the alcohol-associated liver disease mice.
A: Representative microphotographs of sirius-red and α-smooth muscle actin (SMA) staining of the livers in the experimental mice; B: Quantification of sirius-red stained fibrotic area and α-SMA-positive area in high-power field (n = 10); C: Hepatic concentration of hydroxyproline (n = 10); D: Hepatic mRNA level of profibrotic markers (n = 10); E: Hepatic activity of matrix metalloproteinases (MMP)-2, MMP-9, and MMP-13 (n = 10); F: Hepatic level of tissue inhibitor of metalloproteinase 1 (n = 10). Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control for real-time quantitative polymerase chain reaction (D). Quantitative values are indicated as fold changes to the values of non-therapeutic group (B, D and E). Data are the mean ± SD. bP < 0.01 vs non-therapeutic group; cP < 0.05 vs vehicle-treated alcohol-associated liver disease group; dP < 0.01 vs vehicle-treated alcohol-associated liver disease group, significant difference between groups by Student’s t-test. NT: Non-therapeutic group; Veh: Vehicle-treated alcohol-associated liver disease group; EFN-L: Elafiblanor (3 mg/kg/day)-treated alcohol-associated liver disease group; EFN-H: Elafibranor (10 mg/kg/day)-treated alcohol-associated liver disease group; α-SMA: α-smooth muscle actin; MMP: Matrix metalloproteinases; TIMP1: Tissue inhibitor of metalloproteinase 1.
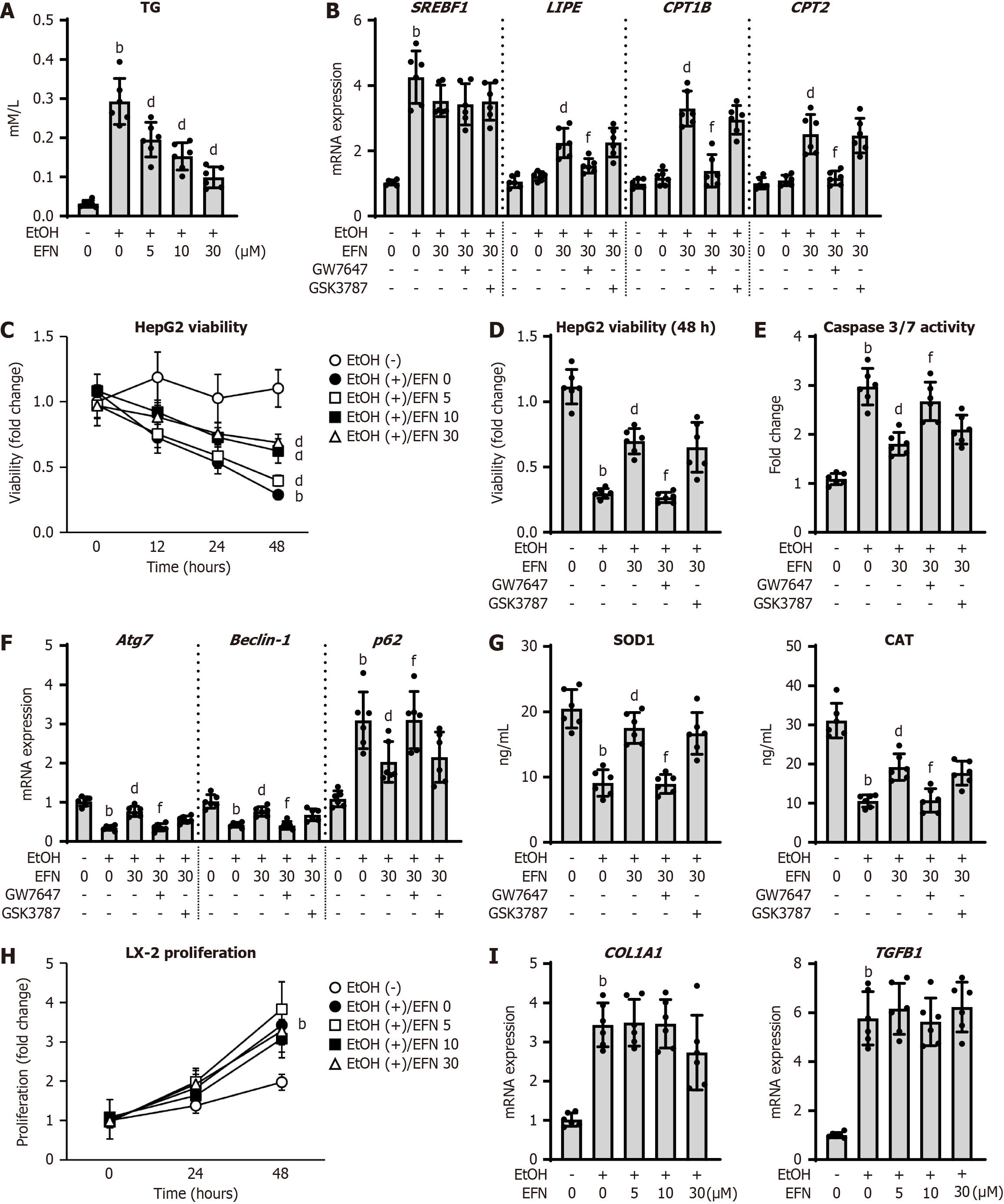
Figure 4 Elafibranor on the ethanol-stimulated human hepatocytes and human hepatic stellate cells.
A: Intracellular triglyceride content in HepG2 cells (n = 6); B: Intracellular mRNA level of the markers related to lipid metabolism in HepG2 cells (n = 6); C: Chronological change in HepG2 cell viability by treatment with ethanol (EtOH) and/or elafibtanor (EFN) (n = 6); D: Effect of a selective antagonists of peroxisome proliferator activated receptor (PPAR)α (GW7647) or PPARδ (GSK3787) on EtOH and EFN-treated HepG2 cell viability (incubation for 48 hours) (n = 6); E: Intracellular caspase 3/7 activity in HepG2 cells (n = 6); F: Intracellular mRNA level of the markers related to autophagy in HepG2 cells (n = 6); G: Intracellular levels of superoxide dismutase 1 and catalase in HepG2 cells (n = 6). HepG2 cells were incubated with (A and C) EtOH (0 or 50 mmol/L) and EFN (0, 5, 10, 30 μM) for 24 hours (A) or 0, 12, 24, and 48 hours (C), EtOH (0 or 50 mmol/L) and EFN (0 or 30 μM) for 48 hour following pretreatment with GW7647 (10 μM) or GSK3787 (10 μM) for 6 hours (B, D-G); H: Chronological change in LX-2 cell proliferation by treatment with EtOH and/or EFN (n = 6); I: Intracellular mRNA level of the profibrotic markers in LX-2 cells (n = 6). LX-2 cells were incubated with EtOH (0 or 50 mmol/L) and EFN (0, 5, 10, and 30 μM) for 0, 24, 48 hours (H) or 24 hours (I). Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control for real-time quantitative polymerase chain reaction (B, F and I). Quantitative values are indicated as fold changes to the values of EtOH (-)/EFN (0 μM)-treated group (B-F, H and I). Data are the mean ± SD. bP < 0.01 vs ethanol (-)/elafibtanor (0 μM)-treated group; dP < 0.01 vs ethanol (+)/elafibtanor (0 μM)-treated group; fP < 0.01 vs ethanol (+)/elafibtanor (30 μM)-treated group. EtOH: Ethanol; EFN: Elafibtanor; SOD: Superoxide dismutase; CAT: Catalase; TG: Triglyceride.
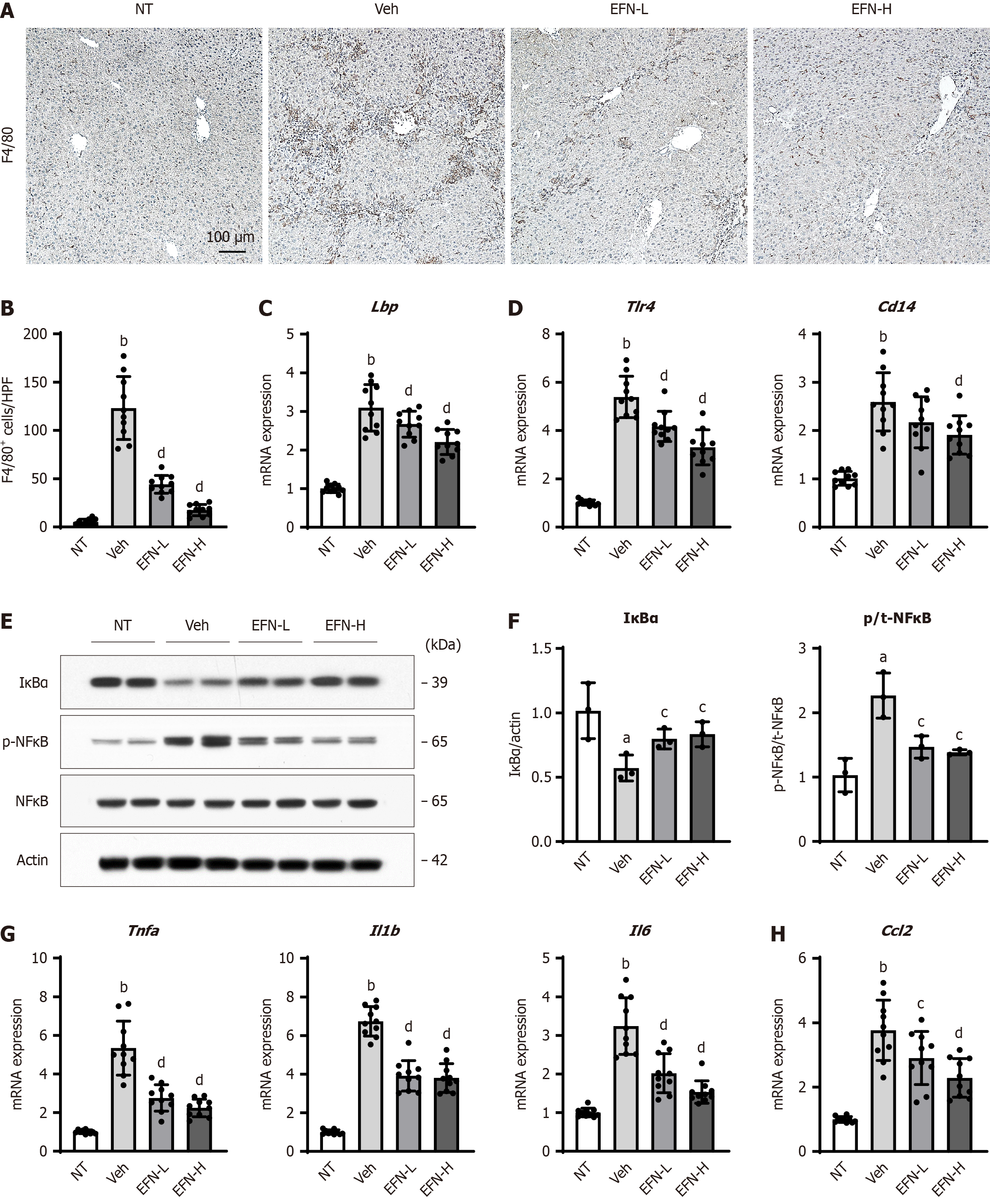
Figure 5 Elafibranor on Kupffer cell-mediated inflammatory response in the alcohol-associated liver disease mice.
A: Representative microphotographs of F4/80 staining of the livers in the experimental mice; B: Quantification of F4/80-positive cells in high-power field (n = 10); C and D: Hepatic mRNA level of lipopolysaccharide-binding protein (C), toll like receptor 4 and CD14 (D) (n = 10); E: Western blot for the protein expression of IκBα, p-nuclear factor kappa B (NFκB) and NF-κB in the liver tissue. Actin was used as an internal control; F: Quantification of the protein level of IκBα and the ratio of NF-κB phosphorylation based on western blotting (n = 10); G and H: Hepatic mRNA level of tumor necrosis factor-alpha, interleukin 1β (Il1b), and Il6 (G), and Ccl2 (H) (n = 10). Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control for real-time quantitative polymerase chain reaction (C, D, G and H). Quantitative values are indicated as fold changes to the values of non-therapeutic group. Data are the mean ± SD. aP < 0.05 vs non-therapeutic group; bP < 0.01 vs non-therapeutic group; cP < 0.05 vs vehicle-treated alcohol-associated liver disease group; dP < 0.01 vs vehicle-treated alcohol-associated liver disease group, significant difference between groups by Student’s t-test. NT: Non-therapeutic group; Veh: Vehicle-treated alcohol-associated liver disease group; EFN-L: Elafiblanor (3 mg/kg/day)-treated alcohol-associated liver disease group; EFN-H: Elafibranor (10 mg/kg/day)-treated alcohol-associated liver disease group; NF-κB: Nuclear factor kappa B; Lbp: Lipopolysaccharide-binding protein; TLR: Toll like receptor; TNF: Tumor necrosis factor; IL: Interleukin.
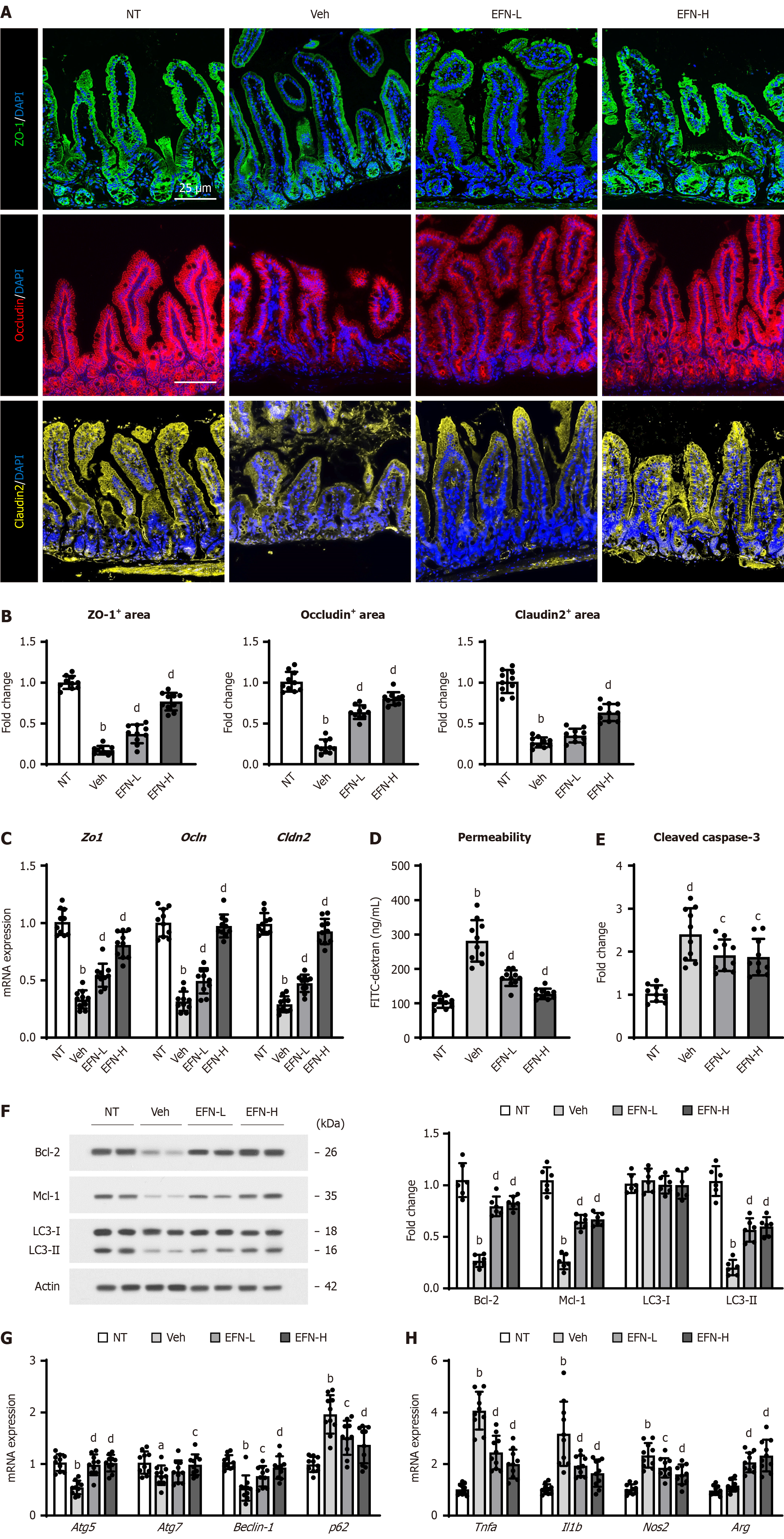
Figure 6 Elafibranor on intestinal barrier function in the alcohol-associated liver disease mice.
A: Representative microphotographs of ileum sections immunofluorescent stained with tight junction proteins (TJPs) including zonula occludens-1 (ZO-1), occludin and claudin-2; B: Quantitation of ZO-1, occludin and claudin-2 immunopositive area in high-power field (n = 10); C: Intestinal mRNA levels of TJPs (n = 10); D: Blood levels of fluorescein isothiocyanate-dextran (4 kDa) 4 hours after oral administration (n = 3); E: Cleaved caspase-3 level in the ileum tissue (n = 10); F: Western blot for the protein expression of Bcl-2, Mcl-1 and LC3-1 and 2 in the ileum tissue. Actin was used as an internal control; G and H: Intestinal mRNA level of the markers related to autophagy (G) and macrophage activation (H) (n = 10). Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control for real-time quantitative polymerase chain reaction (C, G, and H). Quantitative values are indicated as fold changes to the values of non-therapeutic group (B, C, E-H). Data are the mean ± SD. aP < 0.05 vs non-therapeutic group; bP < 0.01 vs non-therapeutic group; cP < 0.05 vs vehicle-treated alcohol-associated liver disease group; dP < 0.01 vs vehicle-treated alcohol-associated liver disease group, significant difference between groups by Student’s t-test. NT: Non-therapeutic group; Veh: Vehicle-treated alcohol-associated liver disease group; EFN-L: Elafiblanor (3 mg/kg/day)-treated alcohol-associated liver disease group; EFN-H: Elafibranor (10 mg/kg/day)-treated alcohol-associated liver disease group; ZO-1: Zonula occludens-1; TNF: Tumor necrosis factor; IL: Interleukin; FITC: Fluorescein isothiocyanate.
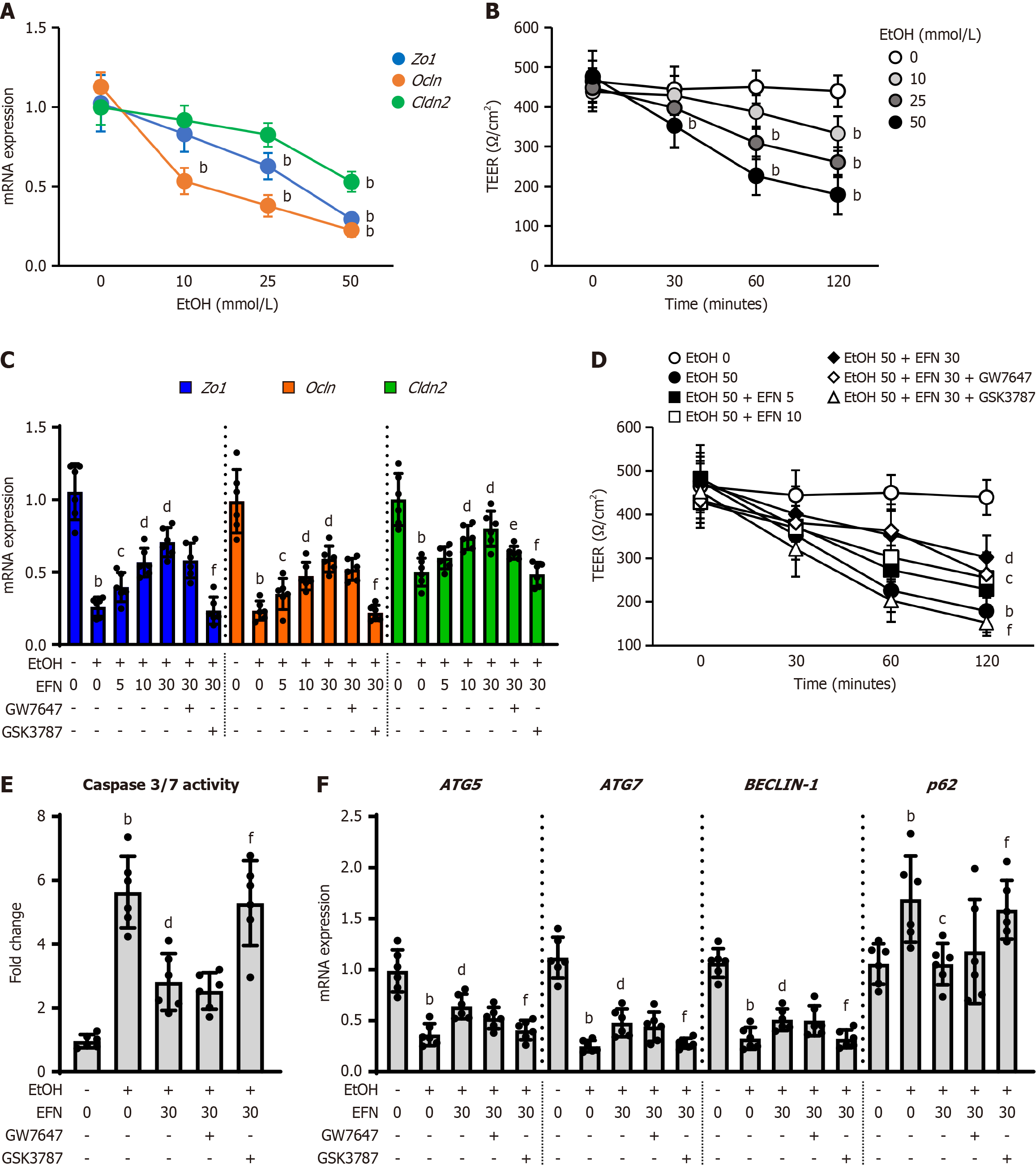
Figure 7 Elafibranor on the ethanol-stimulated human intestinal epithelial cells.
A: Intracellular mRNA levels of tight junction proteins (TJPs) including zonula occludens-1 (ZO-1), Ocln, and Cldn2 in ethanol (EtOH)-stimulated Caco-2 cells (n = 6); B: Integrity of the epithelial cellular barrier in EtOH-stimulated Caco-2 cells determined as transepithelial electrical resistance (TEER) (n = 6). Cells were incubated with different concentration of EtOH (0, 10, 25, and 50 mmol/L) for 120 minutes (A) and 0, 30, 60, and 120 minutes (B); C: Effect of elafibranor (EFN) on the TJPs mRNA expression in the EtOH-stimulated Caco-2 cells (n = 6); D: Effect of EFN on the TEER in the EtOH-stimulated Caco-2 cells (n = 6). Cells were incubated with EtOH (0 or 50 mmol/L) and EFN (0, 5, 10, 30 μM) for 120 minutes (C) or 0, 30, 60, and 120 minutes (D) following pretreatment with GW7647 (10 μM) or GSK3787 (10 μM) for 15 minutes; E: Effect of EFN on the intracellular caspase 3/7 activity in the EtOH-stimulated Caco-2 cells (n = 6); F: Effect of EFN on mRNA expression of the markers related to autophagy in the EtOH-stimulated Caco-2 cells (n = 6). Cells were incubated with EtOH (0 or 50 mmol/L) and EFN (0 or 30 μM) for 48 hours following pretreatment with a peroxisome proliferator activated receptor (PPAR)α antagonist, GW7647 (10 μM) or a PPARδ antagonist, GSK3787 (10 μM) for 6 hours (E and F). Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control for real-time quantitative polymerase chain reaction (A, C, and F). Quantitative values are indicated as fold changes to the values of EtOH (-)/EFN (0 μM)-treated group (A, C, E, and F). Data are the mean ± SD. bP < 0.01 vs ethanol (-)/elafibranor (0 μM)-treated group; cP < 0.05 vs ethanol (+)/elafibranor (0 μM)-treated group; dP < 0.01 vs ethanol (+)/elafibranor (0 μM)-treated group; eP < 0.05 vs ethanol (+)/elafibranor (30 μM)-treated group; fP < 0.01 vs ethanol (+)/elafibranor (30 μM)-treated group. EtOH: Ethanol; EFN: Elafibtanor; TEER: Transepithelial electrical resistance; ZO-1: Zonula occludens-1.
- Citation: Koizumi A, Kaji K, Nishimura N, Asada S, Matsuda T, Tanaka M, Yorioka N, Tsuji Y, Kitagawa K, Sato S, Namisaki T, Akahane T, Yoshiji H. Effects of elafibranor on liver fibrosis and gut barrier function in a mouse model of alcohol-associated liver disease. World J Gastroenterol 2024; 30(28): 3428-3446
- URL: https://www.wjgnet.com/1007-9327/full/v30/i28/3428.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i28.3428